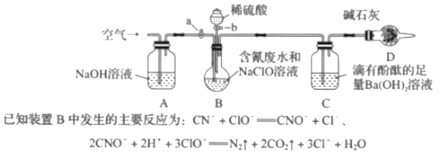
��Ŀ����
����Ŀ����1�����Ѿ���SO2���ʹ����Ϊ0.25 g��L��1��ȡ300.00 mL���Ѿƣ�ͨ���ʵ��ķ���ʹ����SO2ȫ���ݳ�����H2O2����ȫ������ΪH2SO4��Ȼ����0.090 0 mol��L��1NaOH����Һ���еζ���
��д������������ԭ��Ӧ�Ļ�ѧ����ʽ��___________________
������50 mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�����������Һ������________(�����)��
A����10 mL B����40 mL C�� ��10 mL D�� ��40 mL��
�����ζ��յ�ʱPH=8.8����ѡ��_______Ϊָʾ��
�ܵζ��յ����ʱ���ӿ̶��ߣ�����������ʵ��ֵ________������ƫ��������ƫ����������Ӱ��������
��2��ijѧ����0.100molL-1��KOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ��
A����ȡ20mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ���ʹ���촦������Һ��
D��ȡ��KOH��Һע���ʽ�ζ������̶���0������2��3cm ����
E������Һ������0������0�����¿̶ȣ����¶�����
F������ƿ���ڵζ������棬�ñ�KOH��Һ�ζ����յ㲢���¿̶ȡ�
ʵ���� | KOH��Һ��Ũ�ȣ�mol/L�� | �ζ����ʱ��KOH��Һ����������mL�� | ����������Һ�������mL�� |
1 | 0.10 | 22.62 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
����ȷ���������˳���ǣ��������ĸ��д��__________________________
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ______________��������λ��Ч���֣�
���𰸡�SO2+H2O2=H2SO4 D ��̪ ƫ�� B��D��C��E��A��F 0.11mol/L
��������
(1)�ٶ���������л�ԭ�ԣ��ܹ���ʵ���ҷ�Ӧ�������ᣬ�ݴ�д����Ӧ�ķ���ʽ��
�ڸ��ݵζ��ܵĹ����жϵζ�������Һ�������
�۸��ݵζ��յ�ʱ��Һ��pH������ָʾ���ı�ɫ��Χѡ����ȷ��ָʾ����
�ܶ�ȡ�ζ����յ����ʱ�����ӿ̶��ߣ�����ƫС��
(2)���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ��Ȳ�����
���ȷ������ݵ���Ч�ԣ�������ļ��ƽ�������Ȼ�����c(��)= ���㡣
���㡣
(1)��˫��ˮ���������ԣ��ܹ��������������������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O2=H2SO4��
������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶���10�������ζ��ܵ�0�̶����Ϸ���10mL�̶����·�����40mL�п̶ȵ���Һ������ζ���50mL�̶�������Һ�壬��˹��ڵ�Һ�������(50.00mL-10.00mL)=40.00mL���ʴ�ΪD��
�۵ζ��յ�ʱ��Һ�����Ա���ԣ�Ӧ��ѡ���̪��ָʾ��(��̪�ı�ɫ��Χ��8.2��10.0)��
�ܶ�ȡ�ζ����յ����ʱ�����ӿ̶��ߣ�����ƫС�����±�Һ�����ƫС����c(����)= ��֪���ⶨŨ��ƫ�ͣ�����������ʵ��ֵƫ�ͣ�
��֪���ⶨŨ��ƫ�ͣ�����������ʵ��ֵƫ�ͣ�
(2)�ٲ����IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ�������˳��Ϊ��B��D��C��E��A��F��
���������ݾ���Ч��ƽ������V(KOH��Һ)=![]() =22.71mL������c(��)=
=22.71mL������c(��)= =
=![]() ��1.1mol/L��
��1.1mol/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��800��ʱ�����淴Ӧ CO��g��+H2O��g��CO2��g��+H2��g����ƽ�ⳣ�� K=1��800��ʱ�����ijһʱ���ܱ������и���ֵ�Ũ�����������˵����ȷ���ǣ�������
���� | CO | H2O | CO2 | H2 |
Ũ��/molL-1 | 0.002 | 0.003 | 0.0025 | 0.0025 |
A.��ʱƽ�������ƶ�
B.�ﵽƽ�������ѹǿ����
C.�����������ݻ�ѹ��Ϊԭ����һ�룬ƽ����ܻ��������ƶ�
D.����Ӧ������С������ʱ���ﵽ��ѧƽ��״̬