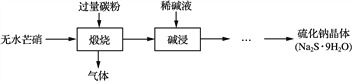
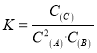��Ŀ����
����Ŀ��ij��ѧʵ��С������ͼ��ʾ��װ����ȡ���������������������������Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�档�����Ҫ����գ�

��1��д��ʵ�����ñ��������ˮ�Ҵ������������Ļ�ѧ����ʽ��_______________��
��2��Ϊʹ��Ӧ���ַ�Ӧ�����´�ʩ����ȷ����________����д��Ӧ��ţ���
��С�����ȣ���������������״̬
���ȴ�����������״̬�����������ȱ��ַ���״̬
��ʹ��ϡ����������
������Ũ����������
��3������������ϵĵ��ܶ�һЩ���������������ռ��к�Ӱ�죬����ԭ��
��___________________________________��
��4��Bͬѧ���ռ����������������뱥��NaHCO3��Һ�У��۲쵽���������ݲ������ɵó��Ľ�����______________���ù����з�����Ӧ�Ļ�ѧ����ʽ��__________________________��
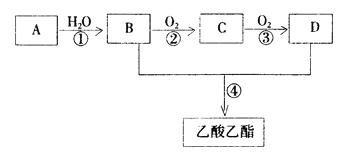
���𰸡� CH3COOH + C2H5OH![]() CH3COOC2H5 + H2O �٢� ʹ���������ò�����ֵ�������ʹ�ռ�������������Ҫ�㹻������ȷ������õ�������� ���������к������� CH3COOH+NaHCO3=CH3COONa+CO2��+H2O
CH3COOC2H5 + H2O �٢� ʹ���������ò�����ֵ�������ʹ�ռ�������������Ҫ�㹻������ȷ������õ�������� ���������к������� CH3COOH+NaHCO3=CH3COONa+CO2��+H2O
����������1��������Ҵ���Ũ���������·���������Ӧ����������������ˮ����Ӧ����ʽΪCH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��2��������Ҵ���Ӧ��Ũ��������������Ӧ���ӷ���Ӧ��С�����ȣ���������������״̬����ѡ�٢���
��3���ӷ�Ӧװ�ó�����Ϊ���������ܾ����������ã�������̫�̣�ʹ���������ò�����ֵ�������ʹ�ռ������٣�����Ҫ�����ȷ������õ����������
��4���������Ա�̼��ǿ��������̼�����Ʒ�Ӧ����Ӧ����ʽΪCH3COOH+NaHCO3=CH3COONa+CO2��+H2O��˵�����������к������ᡣ

����Ŀ��Ԫ��X��Y�� Z��W��Q�� M��Ԫ�����ڱ������λ����ͼ��ʾ������ZԪ����Ŀǰ���ֵķǽ�������ǿ��Ԫ�أ�����˵����ȷ����
X | Y | Z | |
W | Q | M |
A. ��Ӧ�⻯��ķе�Y> Q����ΪY�ķǽ����Ա�Qǿ
B. XM4�ı���ģ��Ϊ![]() �����ȡ����������
�����ȡ����������
C. W������������¿��Ժ�Z��M���⻯�ﷴӦ
D. Z�ĵ����ܽ�Y�ĵ��ʴ������⻯�����û�����
����Ŀ��700��ʱ,���ݻ�Ϊ2L���ܱ������г���һ������CO��H2O,������Ӧ��CO(g)+H2O(g)CO2(g)+H2(g)��Ӧ�����вⶨ�IJ������ݼ��±�(����t2
��Ӧʱ��/min | n(CO)/mol | n(H2O)/mol |
0 | 1.20 | 0.60 |
t1 | 0.80 | |
t2 | 0.20 |
����˵����ȷ����
A. ��Ӧ��t1 min�ڵ�ƽ������Ϊv(H2)=0.40/t1molL1min1
B. ����������������,��ʼʱ�������г���0.60 mol CO��0.10 mol H2O,�ﵽƽ��ʱn(CO2)=0.20 mol
C. ����������������,��ƽ����ϵ����ͨ��0.20 mol H2O,��ԭƽ�����,�ﵽ��ƽ��ʱCO��H2Oת��������
D. �¶�������800�棬�ﵽ��ƽ��ʱn(CO2)=0.34 mol��������ӦΪ���ȷ�Ӧ