��Ŀ����
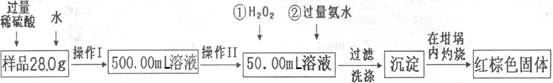
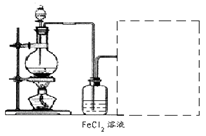
ijʵ��С���ù�ҵ�������壨��Ҫ�ɷ�ΪCu2S��Fe2O3���Ʊ��й����ʣ�������������ͼ��ʾ����ش�
��1������a�Ļ�ѧʽΪ ��
��2����ҺB���������ữ���ټ�������������X�õ���ҺC��д���÷�Ӧ�����ӷ���ʽ ��
��3���Ʊ�����ͭ��Һ�������£�O2��ͭ�ۺ�ϡ����������һ�𣬼�������Ӧ����������ҺD���漴��������ͭ�����������Ϸ���FeSO4��ͭ������������á�
A.��һ����Ӧ�����ӷ���ʽΪ��4Fe2����O2��4H��=4Fe3����2H2O����ڶ�����Ӧ�Ĺ��ӷ���ʽΪ ��
B.�߲����У�����Fe2(SO4)3��ҺʱӦע�� ��
��4���������Ŀ���ǵõ��ϴ�������ͭ��Һ�����������Լ�Y����pH����Ԫ��ȫ������������Ũ��С��10��5mol/L����Ȼ���ٹ��ˣ�Ũ�����ᾧ�ȣ���pH���ٵ���Ϊ_____��
��֪��Ksp[Cu(OH)2]��1��10��22��Ksp[Fe(OH)2] ��1��10��16��Ksp[Fe(OH)3] ��1��10��38
��5����ѧ�ҷ���������Cu2O��̫���������¿��Դ��ֽ�ˮ��
A.һ���¶��£���2L�ܱ������м�������Cu2O��ͨ��2molˮ�������������·�Ӧ��
2H2O(g)��2H2(g)��O2(g) ��H����484kJ/mol
20minĩ���n(O2)��0.16mol�������ʱ��ķ�Ӧ���ʦ�(H2)��_________�����¶��£��˷�Ӧ��ƽ�ⳣ������ʽK��___________________��
B.��֪��2Cu2O(s)��O2(g)��4CuO(s) ��H����292kJ/mol
2C(s)��O2(g)��2CO(g) ��H����221kJ/mol
��д��̿�ۻ�ԭCuO(s)�Ʊ�Cu2O(s)���Ȼ�ѧ����ʽ_________________��

��1������a�Ļ�ѧʽΪ ��
��2����ҺB���������ữ���ټ�������������X�õ���ҺC��д���÷�Ӧ�����ӷ���ʽ ��
��3���Ʊ�����ͭ��Һ�������£�O2��ͭ�ۺ�ϡ����������һ�𣬼�������Ӧ����������ҺD���漴��������ͭ�����������Ϸ���FeSO4��ͭ������������á�
A.��һ����Ӧ�����ӷ���ʽΪ��4Fe2����O2��4H��=4Fe3����2H2O����ڶ�����Ӧ�Ĺ��ӷ���ʽΪ ��
B.�߲����У�����Fe2(SO4)3��ҺʱӦע�� ��
��4���������Ŀ���ǵõ��ϴ�������ͭ��Һ�����������Լ�Y����pH����Ԫ��ȫ������������Ũ��С��10��5mol/L����Ȼ���ٹ��ˣ�Ũ�����ᾧ�ȣ���pH���ٵ���Ϊ_____��
��֪��Ksp[Cu(OH)2]��1��10��22��Ksp[Fe(OH)2] ��1��10��16��Ksp[Fe(OH)3] ��1��10��38
��5����ѧ�ҷ���������Cu2O��̫���������¿��Դ��ֽ�ˮ��
A.һ���¶��£���2L�ܱ������м�������Cu2O��ͨ��2molˮ�������������·�Ӧ��
2H2O(g)��2H2(g)��O2(g) ��H����484kJ/mol
20minĩ���n(O2)��0.16mol�������ʱ��ķ�Ӧ���ʦ�(H2)��_________�����¶��£��˷�Ӧ��ƽ�ⳣ������ʽK��___________________��
B.��֪��2Cu2O(s)��O2(g)��4CuO(s) ��H����292kJ/mol
2C(s)��O2(g)��2CO(g) ��H����221kJ/mol
��д��̿�ۻ�ԭCuO(s)�Ʊ�Cu2O(s)���Ȼ�ѧ����ʽ_________________��
����ȥע���⣬ÿ��2�֣���14�֣���1��SO2(1��)
��2��2Fe2����H2O2��2H����2Fe3����2H2O��4Fe2����O2��4H����4Fe3����2H2O
��3��2Fe3����Cu��2Fe2����Cu2������������Һ�м��������������ֹˮ���Fe2(SO4)3�ܽ���ϡ�����У��ټ�ˮϡ�� ��4��3 ��5��0.008mol/(L��min)����λռ1�֣���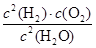 ��1�֣���
��1�֣���
2CuO(s)��C(s)��CO(g)��Cu2O(s) ��H����35.5 kJ/mol
��2��2Fe2����H2O2��2H����2Fe3����2H2O��4Fe2����O2��4H����4Fe3����2H2O
��3��2Fe3����Cu��2Fe2����Cu2������������Һ�м��������������ֹˮ���Fe2(SO4)3�ܽ���ϡ�����У��ټ�ˮϡ�� ��4��3 ��5��0.008mol/(L��min)����λռ1�֣���
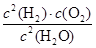 ��1�֣���
��1�֣���2CuO(s)��C(s)��CO(g)��Cu2O(s) ��H����35.5 kJ/mol
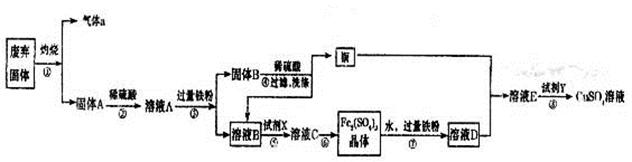
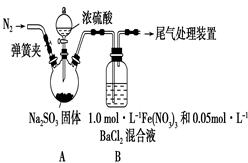
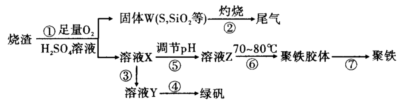
�����������1��Cu2S��������SO2��ͭ����Ӧ�Ļ�ѧ����ʽ��Cu2S��O2
 2Cu��SO2�����������a��SO2��
2Cu��SO2�����������a��SO2����2������A����������ͭ�Ļ�������ϡ��������ܽ��������������������������ܽ�ͭ��������ҺA������������������������ͭ�Լ�����Ļ��Һ��������������ۺ���������������ͭ�����Թ���B��ͭ�����Ļ�����ҺB������������Ҫ�õ����������壬����Ҫ�����������������������������������X������˫��ˮ���������йص����ӷ���ʽ��2Fe2����H2O2��2H����2Fe3����2H2O��4Fe2����O2��4H����4Fe3����2H2O��
��3�����������Ӿ��������ԣ��ܰ�ͭ��������ͭ���ӣ����������ֱ���ԭ�����������ӣ���˵ڶ�����Ӧ�����ӷ���ʽ��2Fe3����Cu��2Fe2����Cu2����������������ˮ��������������������������������Һʱ��Ҫ��ֹ������ˮ�⣬�����ȷ�IJ���Ӧ������������Һ�м��������������ֹˮ���Fe2(SO4)3�ܽ���ϡ�����У��ټ�ˮϡ�͡�
��4����ҺE������ͭ�����������Ļ��Һ�������ܶȻ�������֪��Ҫ�õ�����ͭ��Һ����Ҫ��ȥ�������ӡ������������������ܶȻ���������������ͭ�ģ��������������ܶȻ�����С��������ͭ�ģ�����Ӧ�ü������������������������������ӡ����������������ܶȻ�������֪��Ҫ����pH����Ԫ��ȫ������������Ũ��С��10��5mol/L������Һ�е�OH��Ӧ��Ϊ
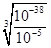 ��10��11mol/L������pH���ٵ���Ϊ3��
��10��11mol/L������pH���ٵ���Ϊ3����5��20minĩ���n(O2)��0.16mol������ݷ���ʽ��֪������������0.16mol��2��0.32mol����Ũ����0.32mol��2L��0.16mol/L����������ķ�Ӧ���ʦ�(H2)��0.16mol/L��20min��0.008mol/(L��min)����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����˸��ݷ���ʽ��֪���¶��£��˷�Ӧ��ƽ�ⳣ������ʽK��
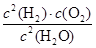 �����ݷ�Ӧ�٣�2Cu2O(s)��O2(g)��4CuO(s) ��H����292kJ/mol�ͷ�Ӧ�ڣ�2C(s)��O2(g)��2CO(g) ��H����221kJ/mol�����ݸ�˹���ɿ�֪�����ڣ��٣���2���õ���Ӧ2CuO(s)��C(s)��CO(g)��Cu2O(s)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H������221kJ/mol��292kJ/mol����2����35.5 kJ/mol��
�����ݷ�Ӧ�٣�2Cu2O(s)��O2(g)��4CuO(s) ��H����292kJ/mol�ͷ�Ӧ�ڣ�2C(s)��O2(g)��2CO(g) ��H����221kJ/mol�����ݸ�˹���ɿ�֪�����ڣ��٣���2���õ���Ӧ2CuO(s)��C(s)��CO(g)��Cu2O(s)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H������221kJ/mol��292kJ/mol����2����35.5 kJ/mol��
��ϰ��ϵ�д�
 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
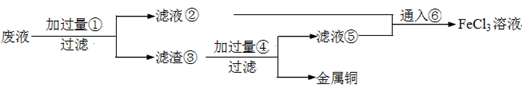
 ����������
���������� ��Һ���
��Һ���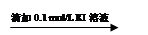 ��Һ��ɫδ��ȥ
��Һ��ɫδ��ȥ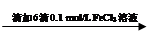 ��Һ���Ա��
��Һ���Ա�� 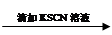 ��Һ���
��Һ��� ��Һ����
��Һ����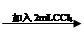 ȡ�ϲ���Һ
ȡ�ϲ���Һ ��Һ���������Թ�2����Һ��ɫ�
��Һ���������Թ�2����Һ��ɫ�