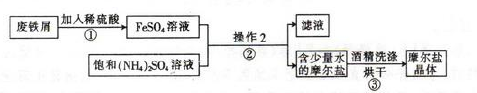
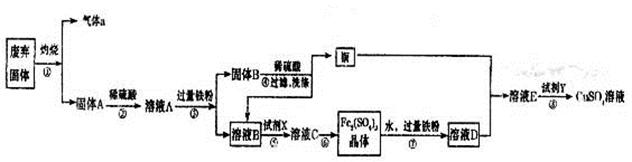
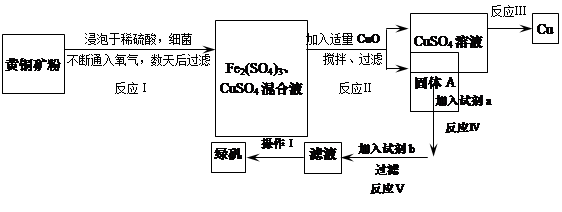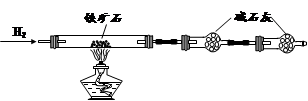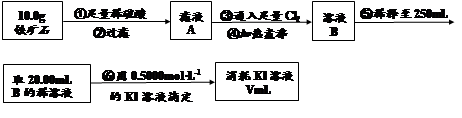��Ŀ����
���ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣�ϸ�ʴҺ���д���CuCl2��FeCl2��FeCl3�������ŷŽ����»�����Ⱦ����Դ���˷ѣ�Ӧ���ǻ������á�������������ʵ���ҽ���ʵ�飺�ӷ�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�á�
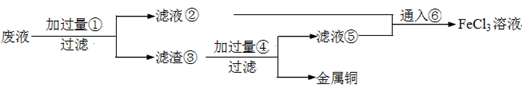
��1��д��FeCl3��Һ��ͭ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2������ϸ�ʴҺ�к���Fe3+��ʵ�������
��3�������ˡ��õ��IJ��������У���ͨ©���� ��
��4����Һ�м�������ٺ�����Ӧ�����ӷ���ʽ��
��5������������ȡ��Һ200 mL�����к�CuCl2 1.5 mol��L��1��FeCl2 3.0 mol��L��1��FeCl3 1.0 mol��L��1����Ҫ��ͭȫ�����գ������Fe�۵�����Ӧ������_____________g�������Ļ�����ȫ��ת��ΪFeCl3��Һ��ͨ��Cl2�����ʵ���������_______________mol��
��6��ij��ѧ��ȤС����������ͼװ����ȡ������ͨ�뵽FeCl2��Һ�л��FeCl3��Һ��
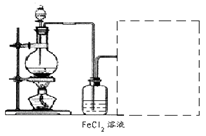
�Ʊ�Cl2�Ļ�ѧ����ʽΪ��
��װ�ò������������������߿��ڻ�����ȱ���֣�����ע�Լ���

��1��д��FeCl3��Һ��ͭ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2������ϸ�ʴҺ�к���Fe3+��ʵ�������
��3�������ˡ��õ��IJ��������У���ͨ©���� ��
��4����Һ�м�������ٺ�����Ӧ�����ӷ���ʽ��
��5������������ȡ��Һ200 mL�����к�CuCl2 1.5 mol��L��1��FeCl2 3.0 mol��L��1��FeCl3 1.0 mol��L��1����Ҫ��ͭȫ�����գ������Fe�۵�����Ӧ������_____________g�������Ļ�����ȫ��ת��ΪFeCl3��Һ��ͨ��Cl2�����ʵ���������_______________mol��
��6��ij��ѧ��ȤС����������ͼװ����ȡ������ͨ�뵽FeCl2��Һ�л��FeCl3��Һ��

�Ʊ�Cl2�Ļ�ѧ����ʽΪ��
��װ�ò������������������߿��ڻ�����ȱ���֣�����ע�Լ���
��1��2FeCl3+ Cu�� 2FeCl2+ CuCl2(2��)
��2��ȡ�����ϸ�ʴҺ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+��2�֣������������𰸣�;
��3�����������ձ�(2��)
��4��2Fe3+ + Fe �� 3Fe2+ (2��) Cu 2+ + Fe �� Fe2+ + Cu(2��)
��5�� 22.4 (2��) 0.6 (2��)
��6��MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O (2��)
MnCl2+Cl2��+2H2O (2��)
 (2��)
(2��)
��2��ȡ�����ϸ�ʴҺ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+��2�֣������������𰸣�;
��3�����������ձ�(2��)
��4��2Fe3+ + Fe �� 3Fe2+ (2��) Cu 2+ + Fe �� Fe2+ + Cu(2��)
��5�� 22.4 (2��) 0.6 (2��)
��6��MnO2+4HCl(Ũ)
 MnCl2+Cl2��+2H2O (2��)
MnCl2+Cl2��+2H2O (2��)  (2��)
(2��)���������������ɷ������������������Ȼ���������������ͭ���������ᣬ�����Ȼ���������������
��1���Ȼ�����ͭ��Ӧ��2FeCl3+ Cu�� 2FeCl2+ CuCl2
��2����Ϊ��ʴҺ�м����������ӣ�Ҳ�����������ӣ����������KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+��
��3�����ݹ���װ�ô��ϵ���Ӧ���У��ձ�����������©����
��4����Һ��CuCl2��FeCl3��Ҫ������Ӧ��2Fe3+ + Fe �� 3Fe2+�� Cu 2+ + Fe �� Fe2+ + Cu��
��5��n��Cu2+��=0.3mol��n��Fe3+��=0.2mol����Ҫn��Fe��=0.3mol+0.1mol=0.4mol��m��Fe��0.4mol��56g/mol=22.4g����Ϻ���Һ��n��Fe2+��=0.8mol������Ҫn��Cl2��=0.4mol��
��6������һ���ö������̺�Ũ������ȡ����Ӧ��Ҫ������������Һ��ȥβ����3+��Fe2+��Cu2+��Cl2�����ʣ�Fe3+�ļ��顢��������ȡ��β�����������˵�װ�ü�β������װ�õ���ƣ����ݷ���ʽ�ļ��㡣

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ