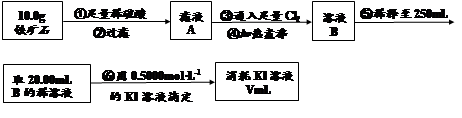��Ŀ����
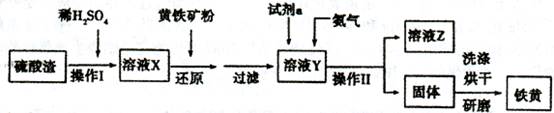
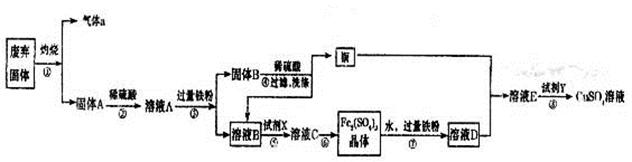
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)����������£�
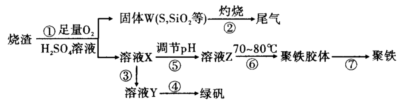
(1)���̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(2)���̢��в�����β����Դ��������Ⱦ����ѡ�������Լ��е� ���ա�
a��ŨH2SO4 b������ˮ c��NaOH��Һ d��Ũ����
(3)���̢��У���Ҫ���������������
(4)���̢ܵ�ʵ�������
(5)���̢��У�����ҺZ���ȵ�70��80�棬Ŀ���� ��
(6)ʵ����Ϊ�ⶨ���õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ��Ʒ2.700 g���ڽ���Ʒ����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4]n����þ�������Ԫ�ص���������Ϊ ��

(1)���̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(2)���̢��в�����β����Դ��������Ⱦ����ѡ�������Լ��е� ���ա�
a��ŨH2SO4 b������ˮ c��NaOH��Һ d��Ũ����
(3)���̢��У���Ҫ���������������
(4)���̢ܵ�ʵ�������
(5)���̢��У�����ҺZ���ȵ�70��80�棬Ŀ���� ��
(6)ʵ����Ϊ�ⶨ���õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ��Ʒ2.700 g���ڽ���Ʒ����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4]n����þ�������Ԫ�ص���������Ϊ ��
��1��4FeS+3O2+6H2SO4=2Fe2(SO4)3+6H2O+4S��2�֣�
��2��c��2�֣� ��3������2�֣�
��4��������Ũ����������ȴ���ᾧ�����ˡ�ϴ�ӣ�4�֣�Ũ������ȴ��д���Բ��۷֣�
��5���ٽ�Fe3+��ˮ�⣨2�֣�
��6��31.11%��3�֣�
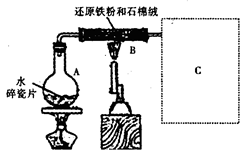
���������
��1�����ݹ���W�ijɷ��д���S���Ƴ�FeS��O2��H2SO4����������ԭ��Ӧ��FeS����ԭ����O2����������������Fe2(SO4)3��S��H2O��
��2����Ⱦ����SO2���ü�Һ���ա�
��3����ҺX�е�����Fe3+���̷��е���ΪFe2+����Ӧ����Fe�ۣ���Fe3+��ԭΪFe2+��
��4�������γɽᾧˮ���������Һ���ܲ�ȡֱ�����ɵİ취��ȡ���塣
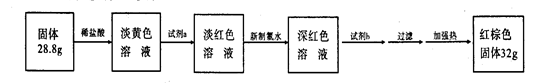
��5�������¶ȣ��ٽ�Fe3+��ˮ�⡣ ��6���۵õ��ij�����BaSO4��n(BaSO4) =
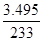 =0.015mol�����ݾ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4] n���Ƴ�n(Fe3+)=0.015mol����m(Fe3+)=0.84g����Ԫ�ص���������:��(Fe)=(0.84g/2.700g)��100%=31.11%��
=0.015mol�����ݾ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4] n���Ƴ�n(Fe3+)=0.015mol����m(Fe3+)=0.84g����Ԫ�ص���������:��(Fe)=(0.84g/2.700g)��100%=31.11%��
��ϰ��ϵ�д�
�����Ŀ