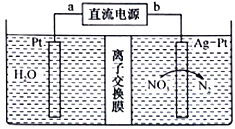
��Ŀ����
����Ŀ����ΪVA��Ԫ�أ�����ұ�����̲����ĺ����ж���ˮ�账�����⡣
I����֪��As(s)+![]() H2(g)+2O2(g)=H3AsO4(s)��H1
H2(g)+2O2(g)=H3AsO4(s)��H1
H2(g)+![]() O2(g)=H2O(l)��H2
O2(g)=H2O(l)��H2
2As(s)+![]() O2(g) =As2O5(s)��H3
O2(g) =As2O5(s)��H3
��ӦAs2O5(s) +3H2O(l)= 2H3AsO4(s)�Ħ�H=_______________��
II.ұ����ˮ����Ԫ����Ҫ��������(H3AsO3)��ʽ���ڣ����û�ѧ�������������Ը�Ũ�Ⱥ����ˮ���乤���������£�
 ��֪����As2S3�������S2-�������·�Ӧ��As2S3(s)+3S2-(aq)
��֪����As2S3�������S2-�������·�Ӧ��As2S3(s)+3S2-(aq)![]() 2AsS33-(aq)��
2AsS33-(aq)��
���������ε��ܽ��Դ�����Ӧ�����Ρ�
��1������������Ԫ�صĻ��ϼ�Ϊ______������ĵ�һ�����뷽��ʽΪ_____________��
��2����һ�����顱��FeSO4��������_______________�� ���������顱��H2O2�뺬�����ʷ�Ӧ�Ļ�ѧ����ʽΪ_________��
III.ȥ��ˮ���е��飬��As(��)ת��ΪAs(��)��Ҳ��ѡ��NaClOʵ�ָ�ת�����о�NaClOͶ������As(��)�����ʵ�Ӱ��õ����½����

��֪��Ͷ��ǰˮ��pH��5.81��0.1mol/LNaClO��ҺpH��10.5����Һ�����������õ������Ǵ����ᡣ�����˽����ԭ����_________________________________________��
���𰸡� 2��H1-3��H2-��H3 +3 H3AsO4![]() H++H2AsO4- ����������S2-��ʹAs2O3(a)+3S2-(aq)
H++H2AsO4- ����������S2-��ʹAs2O3(a)+3S2-(aq)![]() 2AsS32-(aq)ƽ�����ƣ���߳���Ч���� H3AsO3+H2O2=H3AsO4+H2O ���������õ������Ǵ����ᣬNaClO��ҺΪ���ԣ�����������ʱ����Һ������ǿ��NaClO��ҺŨ������ˮ��̶Ƚ��ͣ�����������ɣ�����As(��)�����ʽ���
2AsS32-(aq)ƽ�����ƣ���߳���Ч���� H3AsO3+H2O2=H3AsO4+H2O ���������õ������Ǵ����ᣬNaClO��ҺΪ���ԣ�����������ʱ����Һ������ǿ��NaClO��ҺŨ������ˮ��̶Ƚ��ͣ�����������ɣ�����As(��)�����ʽ���
��������I����As(s)+![]() H2(g)+2O2(g)=H3AsO4(s)��H1����H2(g)+
H2(g)+2O2(g)=H3AsO4(s)��H1����H2(g)+![]() O2(g)=H2O(l)��H2����2As(s)+
O2(g)=H2O(l)��H2����2As(s)+![]() O2(g) =As2O5(s)��H3�����ݸ�˹���ɣ�������2-����3-�۵ã�As2O5(s) +3H2O(l)= 2H3AsO4(s)����H=2��H1-3��H2-��H3���ʴ�Ϊ��2��H1-3��H2-��H3��
O2(g) =As2O5(s)��H3�����ݸ�˹���ɣ�������2-����3-�۵ã�As2O5(s) +3H2O(l)= 2H3AsO4(s)����H=2��H1-3��H2-��H3���ʴ�Ϊ��2��H1-3��H2-��H3��
II.(1)������(H3AsO3)�У���Ԫ�ػ��ϼ�Ϊ-2�ۣ���Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ���Ϊx��+1��3+x+(-2)��3=0��x=+3���ڢ�A��ǽ���Ԫ���γ���ۺ��������ס����γɵĺ����ᶼ�����ᣬˮ��Һ�зֲ����룬���뷽��ʽΪ��H3AsO4H++H2AsO4-���ʴ�Ϊ��+3��H3AsO4H++H2AsO4-��
(2)��һ����������FeSO4�������dz�ȥ�����������ӣ�As2S3+3S2-2AsS32-ʹƽ��������У���߳���Ч������������������H2 O2�뺬�����ʷ���������ԭ��Ӧ������������Ϊ�������Ӧ�Ļ�ѧ����ʽΪ��H2AsO3+H2O2=H3AsO4+H2O���ʴ�Ϊ�����������������ӣ�ʹAs2S3+3S2-2AsS32-ʹƽ��������У���߳���Ч����H2AsO3+H2O2=H3AsO4+H2O��
III.Ͷ��ǰˮ��pH=5.81��0.1mol/LNaClO��ҺpH=10.5����Һ�����������õ������Ǵ����ᣮ�����˽����ԭ���ǣ����������õ������Ǵ����ᣬNaClO��ҺΪ���ԣ�����������ʱ����Һ������ǿ��NaClO��ҺŨ������ˮ��̶Ƚ��ͣ�����������ɣ�����As(��)�����ʽ��ͣ��ʴ�Ϊ�����������õ������Ǵ����ᣬNaClO��ҺΪ���ԣ�����������ʱ����Һ������ǿ��NaClO��ҺŨ������ˮ��̶Ƚ��ͣ�����������ɣ�����As(��)�����ʽ��͡�

����Ŀ�����顢�״����������Դ��
��1����֪�����Ȼ�ѧ����ʽ
��CO2(g)+3H2(g)=CH3OH��g�� +H2O (g)��H= -49.0 kJ/mol
��CH4(g)+2O2(g)=2H2O(g) +CO2(g) ��H= -802.3 kJ/mol
��2H2(g)+O2(g)=2H2O(l) ��H=-571.6 kJ/mol
��H2O(g)=H2O(l) ��H= -44.0 kJ-mol
��CH4(g)+1/2O2(g)=CH3OH(g) ��H =____________��
��2����ҵ�Ϻϳɼ״�����һ�ַ�����2H2(g)+CO(g) ![]() CH3OH(g) ��H��
CH3OH(g) ��H��
һ���¶�����3�������Ϊ1.0 L�ĺ����ܱ������з���������Ӧ��������������±���
���� | �¶�/K | ���ʵ���ʼŨ��/molL-1 | ���ʵ�ƽ��Ũ��/mol.L-1 | ||
c(H2) | c(CO) | c(CH3OH) | c(CH3OH) | ||
I | 400 | 0.20 | 0.10 | 0 | 0.080 |
II | 400 | 0.40 | 0.20 | 0 | |
III | 500 | 0 | 0 | 0.10 | 0.025 |
���÷�Ӧ����H______0(�>����<��)��
���ﵽƽ��ʱ������I��c(CH3OH)_____0.16mol/L(�>�� ��<�� ��=��)��
��400 K ʱ�÷�Ӧ��ƽ�ⳣ��K=________��
(3)���ױ���ij����������CO2��ȱ���Ƽ�����о���������:
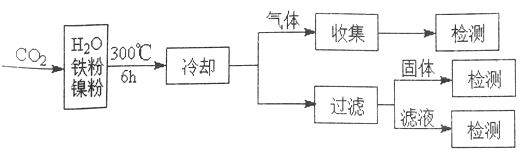
��Ӧ�����������м�CH4��H2����Һ�м�HCOOH�������м����ۺ�Fe3O4��CH4��HCOOH��H2 �IJ��������������Ĺ�ϵ����ͼ��ʾ(�Ǹı�����������������������)���о���Ա����ʵ�����ó�����:HCOOH ��CO2ת��ΪCH4 ���м��壬��:![]()
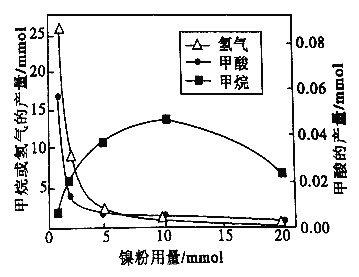
��д������H2�ķ�Ӧ����ʽ_________��
����ͼ��֪��������_______(����ĸ)��
a.��ӦI�Ĵ��� b.��ӦII �Ĵ���
c.��ӦI��II�Ĵ��� d.���Ǵ���
��������������1mmol���ӵ�10mmol����Ӧ���ʵı仯�����______(����ĸ)��
a.��Ӧ1���������ӣ���ӦII�����ʲ���
b.��ӦI�����ʲ��䣬��ӦII����������
c.��ӦI��II�����ʾ�����
d.��ӦI��II�����ʾ����ӣ��ҷ�ӦI���������ӵÿ�
e.��ӦI��II�����ʾ����ӣ��ҷ�ӦII ���������ӵÿ�
f.��ӦI�����ʼ�С����ӦII����������
����Ŀ�������ҹ��Ƽ��ɹ����漰���ʵ�Ӧ���У�������������ѧ�仯����
|
|
|
|
A���״���������������������Դ���� | B��뮡������������̫�����˾۱�ȼ�� | C��ƫ�����������������칬�������Ļ��ȼ�� | D�����ɿ�ȼ����������Ϊ��Դʹ�� |
A. A B. B C. C D. D