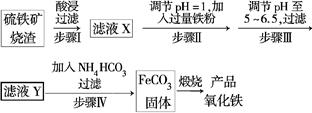
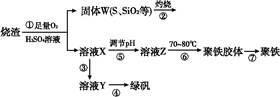
��Ŀ����
ij��ѧС��������װ�ö������仯��������ʽ���̽�����ش��й����⣺
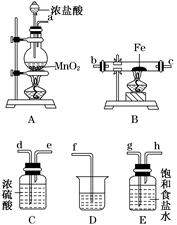
��1����С��ͬѧ���Ƶ�����������������ϳ�ʱ�䣬����ͼ��a����ʾװ�ö�����в�����
��ijͬѧ�IJ����ǣ��ȼн�ֹˮ��K����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������
��
��Ϊ�ﵽʵ��Ŀ�ģ���ȷ�IJ����� ��
B���з�����Ӧ�����ӷ���ʽ�� ��
��2��������װ�õ�ʵ�������ȡA���ڷ�Ӧ���õ���Һ����������С�����ɺ�õ�FeSO4���ٸ������գ��й�װ�úͲ���������ȥ����������º���ɫ���壬���ֽ�ʱ���������尴ͼ��b����ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�����Ʒ����Һ��ɫ��ȥ���ش�
���÷���ʽ��ʾ�Թܼײ�����ɫ������ԭ��
��
��Ϊ��֤����ɫ����ɷ֣��ɽ������²���
��
��ͼ��b���б�װ�õ������� ��
�ܸ���ʵ������д��A������Һ���ɺ��ڸ������շֽ�ʱ��������Ӧ�Ļ�ѧ����ʽ ��
��1����С��ͬѧ���Ƶ�����������������ϳ�ʱ�䣬����ͼ��a����ʾװ�ö�����в�����

��ijͬѧ�IJ����ǣ��ȼн�ֹˮ��K����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������
��
��Ϊ�ﵽʵ��Ŀ�ģ���ȷ�IJ����� ��
B���з�����Ӧ�����ӷ���ʽ�� ��
��2��������װ�õ�ʵ�������ȡA���ڷ�Ӧ���õ���Һ����������С�����ɺ�õ�FeSO4���ٸ������գ��й�װ�úͲ���������ȥ����������º���ɫ���壬���ֽ�ʱ���������尴ͼ��b����ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�����Ʒ����Һ��ɫ��ȥ���ش�
���÷���ʽ��ʾ�Թܼײ�����ɫ������ԭ��
��
��Ϊ��֤����ɫ����ɷ֣��ɽ������²���
��
��ͼ��b���б�װ�õ������� ��
�ܸ���ʵ������д��A������Һ���ɺ��ڸ������շֽ�ʱ��������Ӧ�Ļ�ѧ����ʽ ��
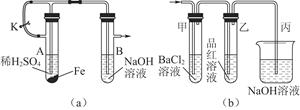
��1����A����Һ����B�У���ʼ������ɫ������Ȼ���Ϊ����ɫ������ɺ��ɫ
�ڴ�ֹˮ��a��ʹA�ܷ�Ӧһ��ʱ����B�е����о������ݲ������ټн�ֹˮ��a
Fe2����2OH��=Fe��OH��2�� ���������кͷ�Ӧд��д���ɣ�
��2����SO3��H2O=H2SO4��
H2SO4��BaCl2=BaSO4����2HCl
��SO3��H2O��BaCl2=BaSO4����2HCl
��ȡ��������������ٵμ�KSCN��Һ���۲쵽��Һ���ɫ
������SO2
��2FeSO4 Fe2O3��SO3����SO2��
Fe2O3��SO3����SO2��
�ڴ�ֹˮ��a��ʹA�ܷ�Ӧһ��ʱ����B�е����о������ݲ������ټн�ֹˮ��a
Fe2����2OH��=Fe��OH��2�� ���������кͷ�Ӧд��д���ɣ�
��2����SO3��H2O=H2SO4��
H2SO4��BaCl2=BaSO4����2HCl
��SO3��H2O��BaCl2=BaSO4����2HCl
��ȡ��������������ٵμ�KSCN��Һ���۲쵽��Һ���ɫ
������SO2
��2FeSO4
 Fe2O3��SO3����SO2��
Fe2O3��SO3����SO2����1����װ��a������ʵ���ԭ��Ϊ��ʹ����ϡ���ᷴӦ����H2��FeSO4����ֹˮ��K��ʹ���ɵ�H2��װ���ڿ���ȫ���ų���ر�ֹˮ�У�H2������ѹ����A���е�FeSO4��Һ��ѹ����B���У�����Fe��OH��2��ɫ����������H2�ı����У��ܱ��ֽϳ�ʱ�䲻��ɫ��
��2���Թܼ��ܲ�����ɫ������˵��һ��������SO3��SO2����BaCl2��Һ��Ӧ������װ�õ������Ǽ���SO2������װ�õ�����������SO2����ֹ��Ⱦ���������⣬����FeSO4����ʱ����SO2��SO3��Fe2O3���ٽ�ϵ����غ��д����Ӧ�Ļ�ѧ����ʽ��
��2���Թܼ��ܲ�����ɫ������˵��һ��������SO3��SO2����BaCl2��Һ��Ӧ������װ�õ������Ǽ���SO2������װ�õ�����������SO2����ֹ��Ⱦ���������⣬����FeSO4����ʱ����SO2��SO3��Fe2O3���ٽ�ϵ����غ��д����Ӧ�Ļ�ѧ����ʽ��

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
 3Fe��4CO2������1 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����________ mol��
3Fe��4CO2������1 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����________ mol��