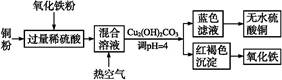
��Ŀ����
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O),��Ҫ�����������¡�
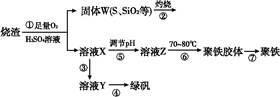
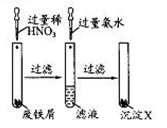
(1)�����̢ڲ���������ͨ��������Һ��,��Һ����ɫ������������
A.Ʒ����Һ B.��ɫʯ����Һ
C.����KMnO4��Һ D.��ˮ
(2)���̢���,FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(3)���̢����������������� ��
(4)���̢���,�����ᾧʱ��ʹ�õ��������ƾ��ơ����ż���,����Ҫ ��
(5)���̢ݵ���pH��ѡ�������Լ��е���������(�����)��
A.ϡ���� B.CaCO3 C.NaOH��Һ
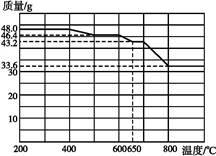
(6)���̢���,����ҺZ���ȵ�70��80 ��,Ŀ������ ��
(7)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص���������,��������ʵ�顣���÷�����ƽ��ȡ2.700 0 g��Ʒ;�ڽ���Ʒ���������������,���������BaCl2��Һ;�۹��ˡ�ϴ�ӡ�����,���صù�������Ϊ3.495 0 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n,��þ�����Ʒ����Ԫ�ص���������Ϊ����������(���������в�����Ԫ�غ���Ԫ��)

(1)�����̢ڲ���������ͨ��������Һ��,��Һ����ɫ������������
A.Ʒ����Һ B.��ɫʯ����Һ
C.����KMnO4��Һ D.��ˮ
(2)���̢���,FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(3)���̢����������������� ��
(4)���̢���,�����ᾧʱ��ʹ�õ��������ƾ��ơ����ż���,����Ҫ ��
(5)���̢ݵ���pH��ѡ�������Լ��е���������(�����)��
A.ϡ���� B.CaCO3 C.NaOH��Һ
(6)���̢���,����ҺZ���ȵ�70��80 ��,Ŀ������ ��
(7)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص���������,��������ʵ�顣���÷�����ƽ��ȡ2.700 0 g��Ʒ;�ڽ���Ʒ���������������,���������BaCl2��Һ;�۹��ˡ�ϴ�ӡ�����,���صù�������Ϊ3.495 0 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n,��þ�����Ʒ����Ԫ�ص���������Ϊ����������(���������в�����Ԫ�غ���Ԫ��)
(1)ACD
(2)4FeS+3O2+6H2SO4 2Fe2(SO4)3+6H2O+4S
2Fe2(SO4)3+6H2O+4S
(3)Fe
(4)����������
(5)C
(6)�ٽ�Fe3+��ˮ��
(7)31.1%
(2)4FeS+3O2+6H2SO4
 2Fe2(SO4)3+6H2O+4S
2Fe2(SO4)3+6H2O+4S(3)Fe
(4)����������
(5)C
(6)�ٽ�Fe3+��ˮ��
(7)31.1%
SO2����Ư����,��ʹƷ����Һ��ɫ,SO2�л�ԭ��,�ܱ�����KMnO4��Һ����ˮ����,��ʹ������ɫ����ҺXΪFe2(SO4)3,����뻹ԭ����Fe3+��ԭΪFe2+,�ֲ�������������,����������ۡ����̢����轫�����Ķ�����pH,��CaCO3�����ᷴӦ����������ˮ��CaSO4����ֹ��Ӧ�ļ������С��ɾ����Ļ�ѧʽ��֪:n(Fe3+)=n(S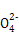 )=
)=
n(BaSO4)=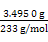 ="0.015" mol,���Ծ�����Ʒ����Ԫ�ص���������=
="0.015" mol,���Ծ�����Ʒ����Ԫ�ص���������=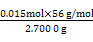 ��100%��31.1%��
��100%��31.1%��
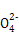 )=
)=n(BaSO4)=
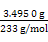 ="0.015" mol,���Ծ�����Ʒ����Ԫ�ص���������=
="0.015" mol,���Ծ�����Ʒ����Ԫ�ص���������=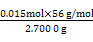 ��100%��31.1%��
��100%��31.1%��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ