ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬±μ÷– Β―ι≤ΌΉςΓΔœ÷œσ”κΫα¬έΕ‘”ΠΙΊœΒ’ΐ»ΖΒΡ «
―Γœν | Β―ι≤ΌΉς | Β―ιœ÷œσ | Ϋα¬έ |
A | “‘Ζ”ΧΣΈΣ÷Η ΨΦΝΘ§”Ο―ΈΥα±ξΉΦ“ΚΒΈΕ®«β―θΜ·ΡΤ»ή“Κ | »ή“Κ”…Κλ…Ϊ±δΈΣ«≥Κλ…Ϊ±ψΝΔΩΧΕΝ ΐ | ≤βΒΟ«β―θΜ·ΡΤ»ή“ΚΒΡ≈®Ε»ΤΪ¥σ |
B | œρΚ§”–Ζ”ΧΣΒΡNa2CO3»ή“Κ÷–Φ”»κ…ΌΝΩBaC12ΙΧΧε | ”–ΑΉ…Ϊ≥ΝΒμ…ζ≥…Θ§»ή“ΚΚλ…Ϊ±δ«≥ | ÷ΛΟς¥ΩΦν»ή“Κ≥ Φν–‘ «”…CO32-Υ°Ϋβ“ΐΤπΒΡ |
C | “Έ¬œ¬Ζ÷±π≤βΕ®NaClO»ή“ΚΓΔCH3COONa»ή“ΚΒΡpH | «Α’Ώ¥σ | Υα–‘ΘΚHClO>CH3COOH |
D | NaHCO3»ή“Κ÷–ΒΈ»κΖ”ΧΣ | »ή“Κ±δΚλ | »θΥαΒΡΥα Ϋ―Έ»ή“ΚΨυ≥ Φν–‘ |
A. A B. B C. C D. D
ΓΨ¥πΑΗΓΩB
ΓΨΫβΈωΓΩAΓΔ»ή“Κ”…Κλ…Ϊ±δΈΣ«≥Κλ…ΪΝΔΦ¥ΕΝ ΐΘ§œϊΚΡΒΡ―ΈΥαΒΡΧεΜΐΩ…ΡήΤΪ–ΓΘ§≤βΒΡ«β―θΜ·ΡΤ»ή“ΚΒΡ≈®Ε»ΤΪ–ΓΘ§Ι A¥μΈσΘΜBΓΔΧΦΥαΡΤ»ή“Κ÷–¥φ‘ΎCO32Θ≠ΘΪH2O![]() HCO3Θ≠ΘΪOHΘ≠Θ§»ή“Κœ‘Φν–‘Θ§ΒΈ»κΖ”ΧΣ»ή“Κœ‘Κλ…ΪΘ§Φ”»κ…ΌΝΩBaCl2»ή“ΚΘ§ΖΔ…ζBa2ΘΪΘΪCO32Θ≠=BaCO3ΓΐΘ§ ΙΤΫΚβœρΡφΖ¥”ΠΖΫœρΫχ––Θ§c(OHΘ≠)Φθ–ΓΘ§Κλ…Ϊ±δ«≥Θ§ΡήΙΜΥΒΟςΫα¬έΘ§Ι B’ΐ»ΖΘΜCΓΔΗυΨί―ΈάύΥ°ΫβΒΡΙφ¬…Θ§ΥαΗυ‘Ϋ»θΘ§Υ°Ϋβ≥ΧΕ»‘Ϋ¥σΘ§NaClO»ή“ΚΒΡpHΉν¥σΘ§ΥΒΟςCH3COOHΥα–‘«Ω”ΎHClOΘ§Ι C¥μΈσΘΜDΓΔ≤Μ «Υυ”–ΒΡ»θΥαΒΡΥα Ϋ―Έ»ή“ΚΨυ≥ Φν–‘Θ§»γNaHSO3»ή“Κ÷–HSO3Θ≠ΒΡΒγάκ¥σ”ΎΤδΥ°ΫβΘ§»ή“Κœ‘Υα–‘Θ§Ι D¥μΈσΓΘ
HCO3Θ≠ΘΪOHΘ≠Θ§»ή“Κœ‘Φν–‘Θ§ΒΈ»κΖ”ΧΣ»ή“Κœ‘Κλ…ΪΘ§Φ”»κ…ΌΝΩBaCl2»ή“ΚΘ§ΖΔ…ζBa2ΘΪΘΪCO32Θ≠=BaCO3ΓΐΘ§ ΙΤΫΚβœρΡφΖ¥”ΠΖΫœρΫχ––Θ§c(OHΘ≠)Φθ–ΓΘ§Κλ…Ϊ±δ«≥Θ§ΡήΙΜΥΒΟςΫα¬έΘ§Ι B’ΐ»ΖΘΜCΓΔΗυΨί―ΈάύΥ°ΫβΒΡΙφ¬…Θ§ΥαΗυ‘Ϋ»θΘ§Υ°Ϋβ≥ΧΕ»‘Ϋ¥σΘ§NaClO»ή“ΚΒΡpHΉν¥σΘ§ΥΒΟςCH3COOHΥα–‘«Ω”ΎHClOΘ§Ι C¥μΈσΘΜDΓΔ≤Μ «Υυ”–ΒΡ»θΥαΒΡΥα Ϋ―Έ»ή“ΚΨυ≥ Φν–‘Θ§»γNaHSO3»ή“Κ÷–HSO3Θ≠ΒΡΒγάκ¥σ”ΎΤδΥ°ΫβΘ§»ή“Κœ‘Υα–‘Θ§Ι D¥μΈσΓΘ

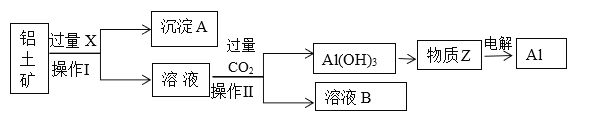
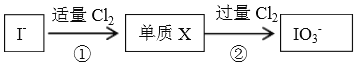
ΓΨΧβΡΩΓΩΦΉ¥ΦΓΔ““¥Φ «…ζΜν÷–≥ΘΦϊ«“”ΟΆΨΙψΖΚΒΡΈο÷ Θ§ΤδΚœ≥…ΖΫΖ®ΚΆ–‘÷ ΨυΨΏ”–―–ΨΩΦέ÷ΒΓΘ
(1)“―÷Σœ¬±μ÷–ΦϋΡή ΐΨίΘ§‘ρΤχΧ§““¥ΦΆξ»Ϊ»Φ…’…ζ≥…CO2ΚΆΥ°’τΤχΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ__________ΓΘ
Μ·―ßΦϋ | C-C | C-H | O-O | H-O | C-O | C-O |
ΦϋΡή/(kJΓΛmol-1) | 348 | 413 | 498 | 463 | 351 | 799 |
(2)œρ“Μ»ίΜΐΩ…±δΒΡΟή±’»ίΤς÷–≥δ»κ1mol CO”κ2 molH2Θ§ΖΔ…ζΖ¥”ΠΘΚCO(g)+2H2(g)![]() CH3OH(g) ΓςH1<0ΓΘCO‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡΤΫΚβΉΣΜ·¬ (a)”κ―Ι«ΩΒΡΙΊœΒ»γΆΦΥυ ΨΓΘ
CH3OH(g) ΓςH1<0ΓΘCO‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡΤΫΚβΉΣΜ·¬ (a)”κ―Ι«ΩΒΡΙΊœΒ»γΆΦΥυ ΨΓΘ
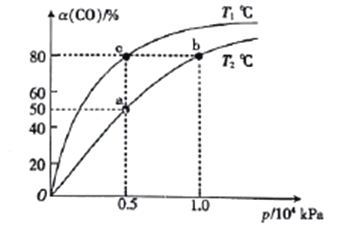
ΔΌaΓΔbΝΫΒψΒΡΖ¥ΈΜΥΌ¬ ΘΚv(b)_____v(a)(ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±Θ§œ¬Ά§)ΓΘ
ΔΎT1____T2ΓΘ
ΔέΗΟΚœ≥…Ζ¥”ΠΒΡΈ¬Ε»“ΜΑψΩΊ÷Τ‘Ύ240ΓΪ270ΓφΘ§―Γ‘ώ¥ΥΖΕΈßΒΡ‘≠“ρΘΚ¥ΥΈ¬Ε»ΖΕΈßœ¬ΒΡ¥ΏΜ·ΦΝΜν–‘ΗΏΘΜ__________________________ΓΘ
ΔήΆΦ÷–aΓΔbΓΔc»ΐΒψΕ‘”ΠΒΡΜ·―ßΤΫΚβ≥ΘΫΧK(a)ΓΔK(b)ΓΔK(c)ΒΡ¥σ–ΓΙΊœΒΈΣ_________ΓΘ
(3)άϊ”ΟΚœ≥…Τχ(÷ς“Σ≥…Ζ÷ΈΣCOΚΆH2)Κœ≥…ΦΉ¥ΦΘ§÷ς“ΣΖΔ…ζ»γœ¬Ζ¥”ΠΘΚ
CO(g)+2H2(g) ![]() CH3OH(g) ΓςH1ΘΜ
CH3OH(g) ΓςH1ΘΜ
CO2(g)+H2(g) ![]() CO(g)+H2O(g) ΓςH2ΘΜ
CO(g)+H2O(g) ΓςH2ΘΜ
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ΓςH3ΓΘ
CH3OH(g)+H2O(g) ΓςH3ΓΘ
…œ ωΖ¥”ΠΕ‘”ΠΒΡΤΫΚβ≥Θ ΐΖ÷±πΈΣK1ΓΔK2ΓΔK3Θ§Τδ÷–K1ΓΔK2ΥφΈ¬Ε»ΒΡ±δΜ·»γΆΦΥυ ΨΓΘ

‘ρΓςH1_____((ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±) ΓςH3Θ§άμ”… «__________________ΓΘ