��Ŀ����
��֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=-99kJ��mol-1.��ش��������⣺
��1����֪�������ȼ����Ϊ296 KJ��mol-1��������S(s)����3 molSO3(g)�ġ�H =
�����ȼ���(��ͼ)��100 mL 0.50 mol/L��CH3COOH��Һ��100 mL 0.55 mol/L NaOH��Һ��ϣ��¶ȴ�298.0 K���ߵ�300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ������)��150.5 J/K����Һ�ܶȾ�Ϊ1 g/mL��������Һ�ı�����c��4.184 J/(g��K)��
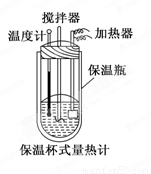
(2) CH3COOH���к��Ȧ�H��_______________________________.
��3��CH3COOH���к��ȵ�����ֵΪ��56.1 kJ/mol������Ϊ(1)�в�õ�ʵ��ֵƫ����ܵ�ԭ���ǣ�����㣩____________________________________________
��1����1185kJ/mol��2�֣� ��2����53.3kJ/mol��2�֣�
��3�������ȼƵı���ƿЧ�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ��
��������
�����������1���������ȼ����Ϊ296 KJ��mol-1����3mol����Sȼ������SO2ʱ�ų���������296 KJ��mol-1��3mol��88kJ�������ɵ�3molSO2��ת��Ϊ3mol��������ʱ�ַų�99kJ��mol-1��3mol��297kJ��������S(s)����3 molSO3(g)�ġ�H����1185kJ/mol��
��2����������ʵ�����0.050mol���������Ƶ����ʵ�����0.055mol����˼�������Ӧ����������������ɵ�ˮ�����ʵ���������0.050molˮ���ɡ���������1molˮ�ķ�Ӧ���ǡ�H�� ��
��
��3�����������֪����Ӧ�зų����������٣���˵����Ӧ������������ʧ�����Կ��ܵ�ԭ���Т����ȼƵı���ƿЧ�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȡ�
���㣺���鷴Ӧ�ȡ��к��ȵ��йؼ����ʵ����������
�����������ڼ��㷴Ӧ��ʱ��Ҳ����ͨ���Ȼ�ѧ����ʽ�����ڸ�˹���ɽ��У��ڷ���ʵ�����ʱ���������ڿ�������Ŀ��ѧ��ֻҪ���жϳ���Ӧ������������ʧ�����Է������������ԭ��

 ��У����ϵ�д�
��У����ϵ�д� 2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺
2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺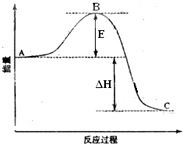 2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ��
2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ��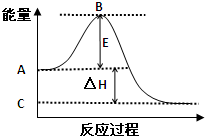 ��1����4�֣�2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol��
��1����4�֣�2SO2��g��+O2��g��?2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol�� ��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��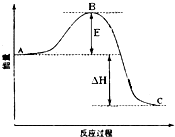 2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺
2SO2��g��+O2��g��=2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3�ġ�H=-99kJ?mol-1����ش��������⣺