��Ŀ����
(15��)ijС������֤Mg�������̼�ķ�Ӧ�������ͼ��ѡ��װ��(���ظ�ʹ��)���и�ʵ�顣���ṩŨ���ᡢϡ���ᡢϡ���ᡢþ�ۡ�����ʯ�������ʯ��ˮ�����͵�NaHCO3��Һ�����͵�Na2CO3��Һ(���ȵ���������ȥ)��
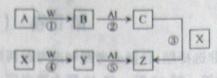
��1���뽫��ѡ�õ�װ�ð�����˳����ϵ���������������У���д��Ӧ������Լ����ɲ�������
��2��A�з�����Ӧ�����ӷ���ʽΪ______________________________________��
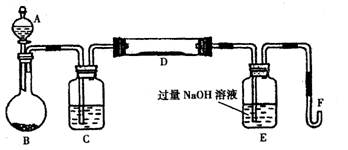
��3����װ��װ�ã�������������ú���ҩƷ���ڼ���C֮ǰӦ���еIJ�����Ŀ����
____________________________________________________________��
��4����Ӧ��Cװ���й۲쵽����Ҫ������_____________________________��

��1���뽫��ѡ�õ�װ�ð�����˳����ϵ���������������У���д��Ӧ������Լ����ɲ�������
| ѡ�õ�װ��(����ţ� | ������Լ� |
| | |
| | |
| | |
| | |
| | |
��3����װ��װ�ã�������������ú���ҩƷ���ڼ���C֮ǰӦ���еIJ�����Ŀ����
____________________________________________________________��
��4����Ӧ��Cװ���й۲쵽����Ҫ������_____________________________��
��1��
��ÿ��1�֣���8�֣���C������������װ�ã������ʯ��ˮ���۷֡���
��2��CaCO3+2H+=Ca2++CO2��+H2O��2�֣�
��3����A�з�Һ©���Ļ������μ�ϡ���ᣬ�����ɵ�CO2�ž�װ���еĿ�������ֹ�����е�������þ��Ӧ����3�֣�
��4������ȼ�գ�����ҫ�۵İ⣬���ɰ�ɫ��ĩ���ܱ��ϸ��ź�ɫ������2�֣����������������ո��֡���
| ѡ�õ�װ�ã�����ţ� | ������Լ� |
| A | ϡ���ᡢ����ʯ |
| B | ���͵�NaHCO3��Һ |
| B | ŨH2SO4 |
| C | þ�� |
| | |
��2��CaCO3+2H+=Ca2++CO2��+H2O��2�֣�
��3����A�з�Һ©���Ļ������μ�ϡ���ᣬ�����ɵ�CO2�ž�װ���еĿ�������ֹ�����е�������þ��Ӧ����3�֣�
��4������ȼ�գ�����ҫ�۵İ⣬���ɰ�ɫ��ĩ���ܱ��ϸ��ź�ɫ������2�֣����������������ո��֡���
��

��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ


 �Թ�B�е�������___________________��
�Թ�B�е�������___________________��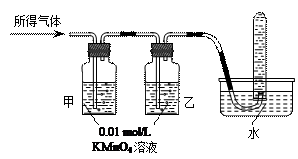

 ��ʱ�õ�b g ��������ʵ���ж��������ת���ʲ�С��_________________��
��ʱ�õ�b g ��������ʵ���ж��������ת���ʲ�С��_________________�� ��
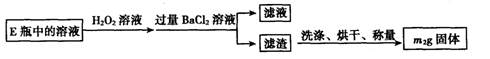
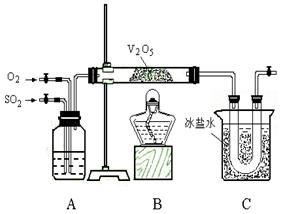
�� ������������ȷ��̼��Ƶ�����������
������������ȷ��̼��Ƶ�����������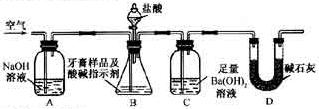
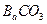 ������ֻҪ�ⶨװ��C������
������ֻҪ�ⶨװ��C������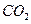 ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______.
ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______.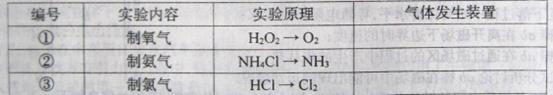
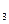 ��
�� װ���Ʊ�O
װ���Ʊ�O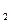 ����ѡ����Լ�Ϊ ���ѧʽ�������������Ʊ�O
����ѡ����Լ�Ϊ ���ѧʽ�������������Ʊ�O ���У�д����Ӧ�ܵ����ӷ���ʽ�� ��
���У�д����Ӧ�ܵ����ӷ���ʽ�� ��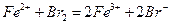 ������100mL��
������100mL��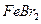 ��Һ��ͨ���״����Cl
��Һ��ͨ���״����Cl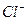 ��
�� �����ʵ���Ũ����ȣ���ͨ��Cl
�����ʵ���Ũ����ȣ���ͨ��Cl