��Ŀ����
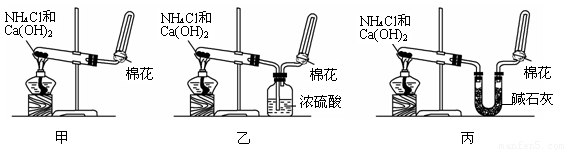
I�ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺
��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_____________________________________________��
��2����λͬѧ������װ����ȡ����ʱ,������ͬѧû���ռ�������������ǵ�ʵ���������ȷ��������Ϊ�ռ�������������Ҫԭ����_____________________________________(�û�ѧ����ʽ��ʾ)��
��3�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ�_______________________
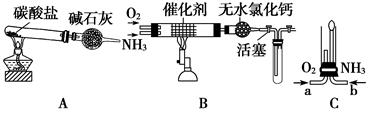
IIijУ��ѧС��ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)���а�����������ʵ�顣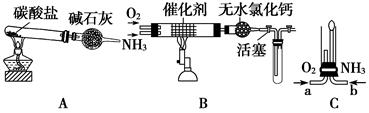
��4����װ��A��ȡ����������İ��������Թ�����̼���Σ���ʯ�ҵ�������__________________________________��
��5���������İ��������������ͨ��װ��B(����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ����������Ļ�ѧ����ʽ��_______________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
1��2NH4Cl + Ca(OH)2 ==CaCl2 + 2NH3��+ 2H2O
(2) 2NH3 + H2SO4 =(NH4)2SO4
��3����ʪ�ĺ�ɫʯ����ֽ���ڹܿڣ�����������
��4������CO2��ˮ����
��5��4NH3 + 5O2 ="==4NO" + 6H2O 2NO + O2 =2NO2
���������������1����λͬѧ���������Ȼ�����������Ʒ�Ӧ�Ʊ��������仯ѧ����ʽΪ
2NH4Cl + Ca(OH)2 ==CaCl2 + 2NH3��+ 2H2O����2�������ܹ���Ũ���ᷴӦ��������ͬѧ�ղ��������������������·�Ӧ��2NH3 + H2SO4 =(NH4)2SO4 ����3�����鰱���Ƿ��ռ����ķ����ǣ���ʪ�ĺ�ɫʯ����ֽ���ڹܿڣ���������������4����̼�������Ȼ�立�Ӧ�����˶�����̼��ˮ�����Լ�ʯ�ҵ�����������CO2��ˮ��������5�����������Ļ�ѧ����ʽ�ǣ�4NH3 + 5O2 ="==4NO" + 6H2O��NO���Ա���������Ϊ����ɫ�Ķ����������仯ѧ����ʽΪ2NO + O2 =2NO2��
���㣺ʵ�����Ʊ�����
���������⿼����ʵ�����Ʊ������Ļ���֪ʶ���Ǹ߿�������ص㣬���ⲻ�ѡ�

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է������������
���� | �״� | ������ | ��������� |
�е㣯�� | 64.7 | 249 | 199.6 |
��Է������� | 32 | 122 | 136 |
��.�ϳɱ���������ֲ�Ʒ
����ƿ�м���12.2g �������20mL �״����ܶ�Լ0.79g/mL�� ����С�ļ���3mL Ũ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��1���÷�Ӧ��Ũ���������?????? ������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ��?????????? ���״�������ԭ��???????????????? ��?
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������?????? ��
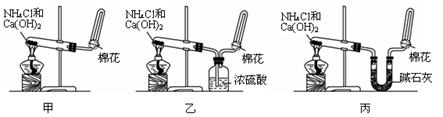
��3���ס��ҡ�����λͬѧ�ֱ��������ͼ����ʵ������ȡ�����������װ�ã��г������ͼ�������������ȥ���������л�����ص㣬��ò���???????? װ�ã����������������������������� ?? 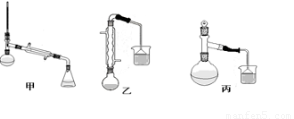
�����ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ���������������ͼ���о��ƣ����������ͼ����ǡ���������������ƣ�����IΪ???? ������IIΪ????? ��

��5����������ͼ�м���Na2CO3��Һ�����Һ©���������ã�Ҫ�õ��л��㣬����������??????????????? ��
��6������������IJ���Ϊ???????? ��