��Ŀ����
��ѧ�о�����������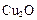 ����Ϊ̫����ֽ�ˮ�Ĵ�����
����Ϊ̫����ֽ�ˮ�Ĵ�����
��������ȡ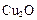 �ķ���
�ķ���
��1����ԭ����̿���ڸ��������»�ԭCuO��
��2����������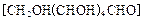 ��ԭ���Ƶ�
��ԭ���Ƶ�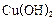 ��д����ѧ����ʽ ��
��д����ѧ����ʽ ��
��3����ⷨ����ӦΪ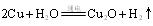 �������������� ��
�������������� ��
��4������ʵ���о����£�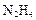 ����ԭ����
����ԭ����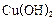 ���Ʊ�����
���Ʊ�����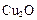 ��ͬʱ�ų�
��ͬʱ�ų�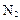 �����Ʒ��Ļ�ѧ����ʽΪ ��
�����Ʒ��Ļ�ѧ����ʽΪ ��
�����Ƶõ�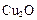 ���д��ֽ�ˮ��ʵ��
���д��ֽ�ˮ��ʵ��
��1��һ���¶��£���2L�ܱ������м�������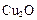 ��ͨ��0��10molˮ������������Ӧ��
��ͨ��0��10molˮ������������Ӧ��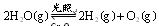 ����H= +484kJ/mol����ͬʱ�β���
����H= +484kJ/mol����ͬʱ�β��� �������±���
�������±���
���㣺ǰ20min�ķ�Ӧ����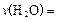 ����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��
����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��
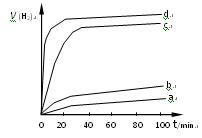
��2�����������ַ����Ƶõ� ��ij��ͬ�����·ֱ��ˮ���ֽ⣬��������������v��ʱ��t�仯��ͼ��ʾ������������ȷ���� ��
��ij��ͬ�����·ֱ��ˮ���ֽ⣬��������������v��ʱ��t�仯��ͼ��ʾ������������ȷ���� ��
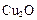 ����Ϊ̫����ֽ�ˮ�Ĵ�����
����Ϊ̫����ֽ�ˮ�Ĵ�������������ȡ
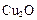 �ķ���
�ķ�����1����ԭ����̿���ڸ��������»�ԭCuO��
��2����������
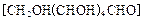 ��ԭ���Ƶ�
��ԭ���Ƶ�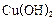 ��д����ѧ����ʽ ��
��д����ѧ����ʽ ����3����ⷨ����ӦΪ
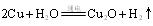 �������������� ��
�������������� ����4������ʵ���о����£�
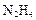 ����ԭ����
����ԭ����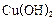 ���Ʊ�����
���Ʊ�����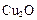 ��ͬʱ�ų�
��ͬʱ�ų�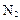 �����Ʒ��Ļ�ѧ����ʽΪ ��
�����Ʒ��Ļ�ѧ����ʽΪ �������Ƶõ�
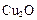 ���д��ֽ�ˮ��ʵ��
���д��ֽ�ˮ��ʵ����1��һ���¶��£���2L�ܱ������м�������
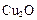 ��ͨ��0��10molˮ������������Ӧ��
��ͨ��0��10molˮ������������Ӧ��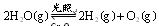 ����H= +484kJ/mol����ͬʱ�β���
����H= +484kJ/mol����ͬʱ�β���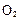 �������±���
�������±���| ʱ��/min | 20 | 40 | 60 | 80 |
| n��O2��/mol | 0��0010 | 0��0016 | 0��0020 | 0��0020 |
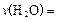 ����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��
����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ����2�����������ַ����Ƶõ�
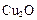 ��ij��ͬ�����·ֱ��ˮ���ֽ⣬��������������v��ʱ��t�仯��ͼ��ʾ������������ȷ���� ��
��ij��ͬ�����·ֱ��ˮ���ֽ⣬��������������v��ʱ��t�仯��ͼ��ʾ������������ȷ���� ��
A��c��d�����Ƶõ�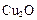 ��Ч����Խϸ� ��Ч����Խϸ� |
B��d�����Ƶõ� ������ʱ��ˮ��ƽ��ת������� ������ʱ��ˮ��ƽ��ת������� |
C����Ч���� �����Ĵ�ϸ��������Ե��й� �����Ĵ�ϸ��������Ե��й� |
| D��Cu2O��ˮ�ֽ�ʱ����Ҫ���˵��¶� |
��2��CH2OH��CHOH��4CHO + 2Cu��OH��2 ��
CH2OH��CHOH��4COOH+Cu2O+2H2O
��3��Cu2O ��4��4Cu��OH��2 + N2H4=2Cu2O + N2 + 6H2O
��1��5��0��10��5 mol��L��1 min��1 0��968 ��2��ACD
CH2OH��CHOH��4COOH+Cu2O+2H2O
��3��Cu2O ��4��4Cu��OH��2 + N2H4=2Cu2O + N2 + 6H2O
��1��5��0��10��5 mol��L��1 min��1 0��968 ��2��ACD
��

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ
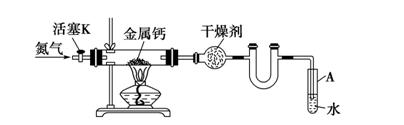
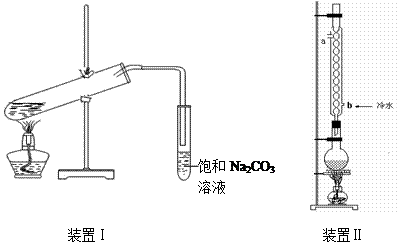
 ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������
��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������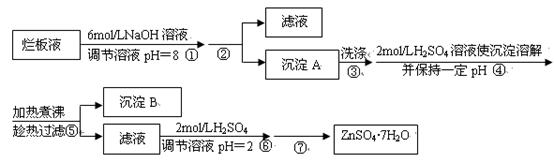
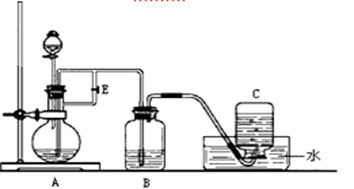
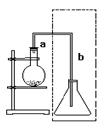
 Ӧ�õ���b���ڿɼ�������������b��ĩ�˸���ƿ��Һ�汣��һ�ξ����Ŀ����___________________��
Ӧ�õ���b���ڿɼ�������������b��ĩ�˸���ƿ��Һ�汣��һ�ξ����Ŀ����___________________��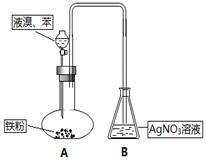
 ���ϲ����У���֤������ϴ�Ӹɾ��ķ�����_______________________________________��
���ϲ����У���֤������ϴ�Ӹɾ��ķ�����_______________________________________��