��Ŀ����
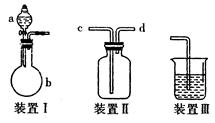
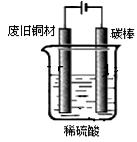
��9�֣���ͼװ�â���ʵ���������������ij���װ�ã�
��װ��1���Թܿ��Ƿ�Ҫ��������
ʵ�������õ�ijЩ�Լ������������������£�
�ش��������⣺
��1�������CH3CO18OH��CH3CH2OH��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ���ڷ�Ӧ����������б��18O��λ�ã� ��ŨH2SO4�������� ��
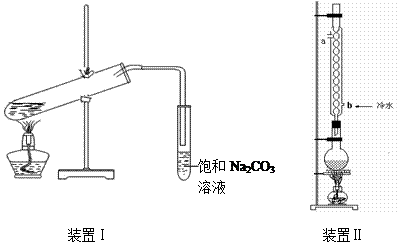
��2��Ҫ ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������
��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ��������� �������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
�������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
��3������װ�â��������������IJ��ʣ���ϱ����е����ݣ�˵����װ�ÿ���������������ʵ�ԭ�� ��

��װ��1���Թܿ��Ƿ�Ҫ��������
ʵ�������õ�ijЩ�Լ������������������£�
| �� �� |
| �е�/�� | �ܶ�/g��cm��3 | ||
| �� �� | ��114 | 78 | 0.789 | ||
| �� �� | 16.6 | 117.9 | 1.05 | ||
| �������� | ��83.6 | 77.5 | 0.900 | ||
| 98%H2SO4 | 10 | 338 | 1.84 |
�ش��������⣺
��1�������CH3CO18OH��CH3CH2OH��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ���ڷ�Ӧ����������б��18O��λ�ã� ��ŨH2SO4�������� ��
��2��Ҫ
 ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������
��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ��������� �������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
�������ã� ��������Ȼ�����_____�ڣ���ϡ����¡�����������3������װ�â��������������IJ��ʣ���ϱ����е����ݣ�˵����װ�ÿ���������������ʵ�ԭ�� ��
��9�֣���1��CH3CO18OH+CH3CH2OH CH3COOCH2CH3+H218O ��2�֣���
CH3COOCH2CH3+H218O ��2�֣���
��������ˮ����2�֣�
��2����Һ©����1�֣����ֲ㣨1�֣����ϣ�1�֣���
��3���ɱ������ݿ�֪���Ҵ�����������������ķе������ڼ��ȹ����У����߾��ӷ�����������װ�â��ʹ��Ӧ��������������ʹ��Ӧ���ַ�Ӧ��������������IJ��ʡ���2�֣�
 CH3COOCH2CH3+H218O ��2�֣���
CH3COOCH2CH3+H218O ��2�֣�����������ˮ����2�֣�
��2����Һ©����1�֣����ֲ㣨1�֣����ϣ�1�֣���
��3���ɱ������ݿ�֪���Ҵ�����������������ķе������ڼ��ȹ����У����߾��ӷ�����������װ�â��ʹ��Ӧ��������������ʹ��Ӧ���ַ�Ӧ��������������IJ��ʡ���2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 �� ��
�� �� 1000mL����ƿ D ������
1000mL����ƿ D ������ 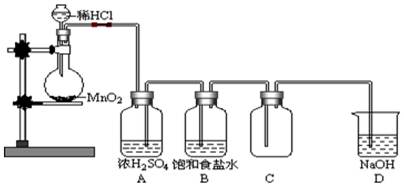
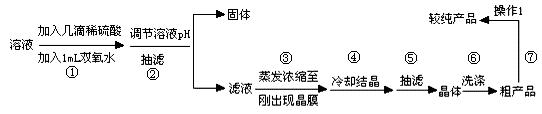
 ��װ��ͼ�еĴ����м�������
��װ��ͼ�еĴ����м�������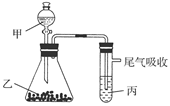
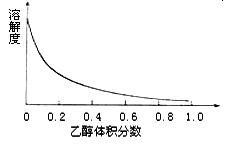

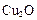 ����Ϊ̫����ֽ�ˮ�Ĵ�����
����Ϊ̫����ֽ�ˮ�Ĵ�����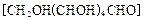 ��ԭ���Ƶ�
��ԭ���Ƶ�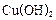 ��д����ѧ����ʽ ��
��д����ѧ����ʽ ��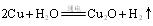 �������������� ��
�������������� ��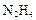 ����ԭ����
����ԭ����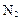 �����Ʒ��Ļ�ѧ����ʽΪ ��
�����Ʒ��Ļ�ѧ����ʽΪ ��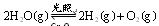 ����H= +484kJ/mol����ͬʱ�β���
����H= +484kJ/mol����ͬʱ�β���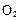 �������±���
�������±���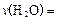 ����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��
����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��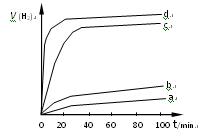
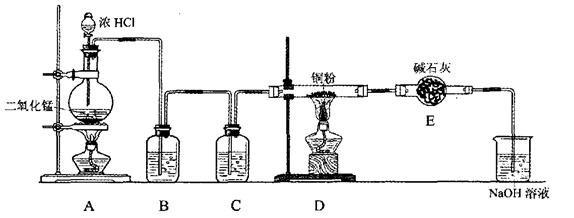 �ش��������⣺
�ش��������⣺ ��8.7gMnO2������Ũ���ᷴӦ���ɱ���µ����� L��
��8.7gMnO2������Ũ���ᷴӦ���ɱ���µ����� L��