��Ŀ����
15��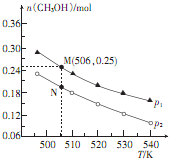 ����Ȼ��Ϊԭ�Ϻϳɼ״������ķ�����ˮú����Ŀǰ���ڿ�����ֱ��������
����Ȼ��Ϊԭ�Ϻϳɼ״������ķ�����ˮú����Ŀǰ���ڿ�����ֱ����������I���й��Ȼ�ѧ����ʽ���£�
ˮú������
CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����H1=-35.4kJ•mol-1
CO��g��+2H2��g��?CH3OH��g����H2=-90.1kJ•mol-1��
ֱ����������
2CH4��g��+O2��g��?2CH3OH��g����H3=-251kJ•mol-1��
��2����ҵ����������̼������Ҳ�ɺϳɼ״���CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H�����ܱ�������Ͷ��1molCO2��2.75mol H2�ڲ�ͬ�����·�����Ӧ��ʵ����ƽ��ʱ�״������ʵ������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
�ٶ�����̼�ϳɼ״�����Ӧ�ġ�H�����������������=������ͬ����
��M��N����ʱ��ѧ��Ӧ���ʣ�v��N����v��M����
��Ϊ���CO2��ת���ʳ��ɸı��¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ��������̼��
��ͼ��M��ʱ���������Ϊ10L����N���Ӧ��ƽ�ⳣ��K=1.04������ֵ������2λС������
��3��һ�������£����ݻ������ij�ܱ������м���a molCO2��b mol H2������ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g������ʹ������Ӧ������CO2���������Ϊ�㶨ֵ����a��b�Ĺ�ϵ��a=b��
���� ��1����CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����H1=-35.4kJ•mol-1
��CO��g��+2H2��g��?CH3OH��g����H2=-90.1kJ•mol-1��
�١�2+2���ڵõ�2CH4��g��+O2��g��?2CH3OH��g�������ݸ�˹�����������ʱ伴�ɣ�
��2������ͼ��֪��ѹǿһ��ʱ���¶�Խ�ߣ�CH3OH�����ʵ���ԽС��˵�������¶�ƽ�����淴Ӧ�����ƶ���
������ӦΪ���������С�ķ�Ӧ���¶�һ��ʱ������ѹǿ��ƽ��������Ӧ�����ƶ����״������ʵ��������ͼ��֪��P1��P2������ѹǿ�Է�Ӧ���ʵ�Ӱ����⣻
���ڿ��淴Ӧ�У�����һ�ַ�Ӧ��Ũ�ȿ��������һ��Ӧ���ת���ʣ�
��ͼ��M��ʱ���������Ϊ10L�����M��������֪��M���CH3OH�����ʵ���Ϊ0.25mol��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��ʼ��mol/L�� 0.1 0.275 0 0
ת����mol/L��0.025 0.075 0.025 0.025
ƽ�⣨mol/L�� 0.075 0.2 0.025 0.025
����K=$\frac{c��{H}_{2}O��•c��C{H}_{3}OH��}{{c}^{3}��{H}_{2}��•c��C{O}_{2}��}$���㣻
��3����ת���Ķ�����̼�����ʵ���Ϊx��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����
��ʼ a b 0 0
ת�� x 3x x x
ƽ�� a-x b-3x x x
��CO2���������Ϊ$\frac{a-x}{a-x+b-3x+x+x}$=$\frac{a-x}{a+b-2x}$���ݴ����ۣ�
��� �⣺��1������CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����H1=-35.4kJ•mol-1
��CO��g��+2H2��g��?CH3OH��g����H2=-90.1kJ•mol-1��
�١�2+2���ڵõ�2CH4��g��+O2��g��?2CH3OH��g�������ԡ�H=2��-35.4kJ•mol-1��+2��-90.1kJ•mol-1��=-251kJ•mol-1���ʴ�Ϊ��-251��
��2������ͼ��֪��ѹǿһ��ʱ���¶�Խ�ߣ�CH3OH�����ʵ���ԽС��˵�������¶�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ����H��0��
�ʴ�Ϊ������
������ӦΪ���������С�ķ�Ӧ���¶�һ��ʱ������ѹǿ��ƽ��������Ӧ�����ƶ����״������ʵ�������ѹǿP1��P2��ѹǿ����Ӧ����Ҳ��������v��N����v��M����
�ʴ�Ϊ������
���ڿ��淴Ӧ�У�����һ�ַ�Ӧ��Ũ�ȿ��������һ��Ӧ���ת���ʣ�����Ϊ���CO2��ת���ʳ��ɸı��¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ�������������ʵ����ȶ�����̼���ʵ�����ֵ���ʴ�Ϊ��������̼�ȣ�
��ͼ��M��ʱ���������Ϊ10L�����M��������֪��M���CH3OH�����ʵ���Ϊ0.25mol��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��ʼ��mol/L�� 0.1 0.275 0 0
ת����mol/L��0.025 0.075 0.025 0.025
ƽ�⣨mol/L�� 0.075 0.2 0.025 0.025
K=$\frac{c��{H}_{2}O��•c��C{H}_{3}OH��}{{c}^{3}��{H}_{2}��•c��C{O}_{2}��}$=$\frac{0.025��0.025}{0��{2}^{3}��0.075}$=1.04��
�ʴ�Ϊ��1.04��
��3����ת���Ķ�����̼�����ʵ���Ϊx��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����
��ʼ a b 0 0
ת�� x 3x x x
ƽ�� a-x b-3x x x
��CO2���������Ϊ$\frac{a-x}{a-x+b-3x+x+x}$=$\frac{a-x}{a+b-2x}$��Ҫʹ$\frac{a-x}{a+b-2x}$Ϊ�㶨��ֵ����a=b��
�ʴ�Ϊ��a=b��
���� ��������ƴ������Ŀ���漰ԭ��ء���Ӧ�ȼ��㡢��ѧƽ�ⳣ������ѧƽ��Ӱ�����ء��л����ƶϵȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

 ������и��⣺
������и��⣺��1����֪���з�Ӧ���Ȼ�ѧ����ʽ��
��6C ��s��+5H2��g��+3N2��g��+9O2��g��=2C3H5��ONO2��3��l����H1��
��2H2��g��+O2 ��g��=2H2O��g����H2��
��C��s��+O2��g���TCO2��g����H3��
��Ӧ4C3H5��ONO2��3��l��=12CO2��g��+10H2O��g��+O2��g��+6N2��g���ġ�HΪ12��H3+5��H2-2��H1��
��2�������Ϊ10L�ĺ����ܱ�������ͨ��3mol X����һ���¶��·������·�Ӧ��2X��g��?Y��g��+az��g������5min��Ӧ�ﵽ��Ӧ�ȣ����ﵽƽ��״̬����
��ƽ��ʱ����������ڵ�ѹǿΪ��ʼʱ��1.2������ʱX�����ʵ���Ũ��Ϊ0.24mol•L-1����ʽ��a=3����Y��ʾ�ķ�Ӧ����Ϊ0.006mol•L-1•min-1��
����������Ӧ�ڼס��ҡ��������ĸ�ͬ�����ܱ������н��У���ͬһʱ���ڲ�������ڵķ�Ӧ���������ʾ��
| ���� | ��Ӧ���� | ���� | ��Ӧ���� |
| �� | v��X��=3.5mol•L-1•min-1 | �� | v��Y��=2 mol•L-1•min-1 |
| �� | v��Z��=4.5mol•L-1•min-1 | �� | v��X��=0.075mol•L-1•s-1 |
��3��������������Դ���������й㷺��;��������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4��װ����ͼ��ʾ��
���������ĵ缫��ӦʽΪ��Fe-6e-+8OH-=FeO42-+4H2O��
������ĤΪ�����ӽ���Ĥ����OH-���������ƶ��������ң���
�ۼ�����ǰ������仯���Բ��ƣ���ȥ��Ĥ��Ϻ���ԭ��Һ�Ƚ�pH���ͣ����ߡ����ͻ䣩
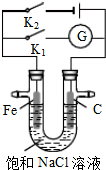
| A�� | K1�պϣ������Ϸ����ķ�ӦΪ2H++2e-��H2�� | |
| B�� | K1�պϣ�ʯī����Χ��ҺpH���� | |
| C�� | K2�պϣ��������ᱻ��ʴ��������ӵ��������������� | |
| D�� | K2�պϣ���·��ͨ��0.4NA������ʱ������������4.48L���� |
| A�� | �������������ᷴӦ OH-+H+=H2O | |
| B�� | ��ϡ����������� Fe2O3+6H+=2Fe3++3H2O | |
| C�� | ʳ��ˮ�еμ���������Һ Cl-+Ag+=AgCl�� | |
| D�� | ��������������ͭ��Һ��Ӧ Ba2++SO42-=BaSO4�� |

