��Ŀ����
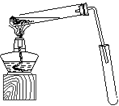
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ���1��д��ʵ�����ñ��������ˮ�Ҵ������������Ļ�ѧ����ʽ��______��
��2��Ϊʹ��Ӧ���ַ�Ӧ�����´�ʩ����ȷ����______����д��Ӧ��ţ���
����С�����ȣ���������������״̬ ���ȴ�����������״̬�����������ȱ��ַ���״̬ ��ʹ��ϡ���������� ������Ũ����������
��3������������ϵĵ��ܶ�һЩ���������������ռ��к�Ӱ�죬����ԭ��
��______��
��4��Aͬѧ���ռ����������������뺬��������̪��NaOH��Һ�в���ˮԡ�����ȣ�������Һ�ĺ�ɫ��dz���ɴ˵ó����������к�������Ľ��ۣ�����Ϊ��һ������ȷ��Ϊʲô��
��______��
��5��Bͬѧ���ռ����������������뱥��NaHCO3��Һ�У��۲쵽���������ݲ������ɵó��Ľ�����______���ù����з�����Ӧ�Ļ�ѧ����ʽ��______��
��6��Cͬѧ���ռ��������������������뱥��Na2CO3��Һ�У������ݲ��������ǵó������������в�������Ľ��ۣ�����������ѧ֪ʶ�����۸�ͬѧ�Ľ����Ƿ���ȷ��
�ҵ������ǣ�______��

���𰸡���������1����������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��2��������Ҵ���Ӧ��Ũ��������������Ӧ���ӷ���Ӧ��С�����ȣ���������������״̬��
��3���ӷ�Ӧװ�ó�����Ϊ���������ܾ����������ã�
��4����������������������Һ����ȫˮ�⣬��Һ���Լ�����
��5�����������������ӷ�������������̼�����Ʒ�Ӧ��
��6������̼���Ʒ�Ӧ������̼�����ƣ����ỹ��ʣ������̼�����Ʒ�Ӧ���ɶ�����̼��
����⣺��1��������Ҵ���Ũ���������·���������Ӧ����������������ˮ����Ӧ����ʽΪCH3CH2OH+CH3COOH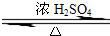 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3CH2OH+CH3COOH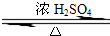 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��2��������Ҵ���Ӧ��Ũ��������������Ӧ���ӷ���Ӧ��С�����ȣ���������������״̬����ѡ���٢ܣ�
��3���ӷ�Ӧװ�ó�����Ϊ���������ܾ����������ã�������̫�̣�ʹ���������ò�����ֵ�������ʹ�ռ������٣�����Ҫ�����ȷ������õ����������
�ʴ�Ϊ��ʹ���������ò�����ֵ�������ʹ�ռ������٣�����Ҫ�����ȷ������õ����������
��4�������������ڼ��������»ᷢ��ˮ�⣬���ɵ�����Ҳ���к�NaOH�Ӷ�ʹ��̪��ɫ���ʷ�̪��ɫ���ܿ϶��Ǻ���������ɵģ�
�ʴ�Ϊ������ȷ�����������ڼ��������»ᷢ��ˮ�⣬���ɵ�����Ҳ���к�NaOH�Ӷ�ʹ��̪��ɫ���ʷ�̪��ɫ���ܿ϶��Ǻ���������ɵģ�
��5���������Ա�̼��ǿ��������̼�����Ʒ�Ӧ����Ӧ����ʽΪCH3COOH+NaHCO3=CH3COONa+CO2��+H2O��˵�����������к������ᣬ
�ʴ�Ϊ�����������к������CH3COOH+NaHCO3=CH3COONa+CO2��+H2O��
��6�������Na2CO3��Ӧ��������NaHCO3�����ų����ݣ���Na2CO3ȫ��ת��ΪNaHCO3���������NaHCO3��Ӧ����CO2���������ݣ����ԣ�û�����ݲ�����������˵�����в��������ᣮ
�ʴ�Ϊ������ȷ���������Na2CO3��Ӧ��������NaHCO3�����ų����ݣ���Na2CO3ȫ��ת��ΪNaHCO3���������NaHCO3��Ӧ����CO2���������ݣ����ԣ�û�����ݲ�����������˵�����в��������ᣮ
���������⿼�������������Ʊ����ѶȲ���ע������֪ʶ�Լ������᷽����ƽ������ۣ�
��2��������Ҵ���Ӧ��Ũ��������������Ӧ���ӷ���Ӧ��С�����ȣ���������������״̬��
��3���ӷ�Ӧװ�ó�����Ϊ���������ܾ����������ã�
��4����������������������Һ����ȫˮ�⣬��Һ���Լ�����
��5�����������������ӷ�������������̼�����Ʒ�Ӧ��
��6������̼���Ʒ�Ӧ������̼�����ƣ����ỹ��ʣ������̼�����Ʒ�Ӧ���ɶ�����̼��
����⣺��1��������Ҵ���Ũ���������·���������Ӧ����������������ˮ����Ӧ����ʽΪCH3CH2OH+CH3COOH
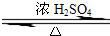 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+CH3COOH
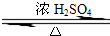 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����2��������Ҵ���Ӧ��Ũ��������������Ӧ���ӷ���Ӧ��С�����ȣ���������������״̬����ѡ���٢ܣ�
��3���ӷ�Ӧװ�ó�����Ϊ���������ܾ����������ã�������̫�̣�ʹ���������ò�����ֵ�������ʹ�ռ������٣�����Ҫ�����ȷ������õ����������
�ʴ�Ϊ��ʹ���������ò�����ֵ�������ʹ�ռ������٣�����Ҫ�����ȷ������õ����������
��4�������������ڼ��������»ᷢ��ˮ�⣬���ɵ�����Ҳ���к�NaOH�Ӷ�ʹ��̪��ɫ���ʷ�̪��ɫ���ܿ϶��Ǻ���������ɵģ�
�ʴ�Ϊ������ȷ�����������ڼ��������»ᷢ��ˮ�⣬���ɵ�����Ҳ���к�NaOH�Ӷ�ʹ��̪��ɫ���ʷ�̪��ɫ���ܿ϶��Ǻ���������ɵģ�
��5���������Ա�̼��ǿ��������̼�����Ʒ�Ӧ����Ӧ����ʽΪCH3COOH+NaHCO3=CH3COONa+CO2��+H2O��˵�����������к������ᣬ
�ʴ�Ϊ�����������к������CH3COOH+NaHCO3=CH3COONa+CO2��+H2O��
��6�������Na2CO3��Ӧ��������NaHCO3�����ų����ݣ���Na2CO3ȫ��ת��ΪNaHCO3���������NaHCO3��Ӧ����CO2���������ݣ����ԣ�û�����ݲ�����������˵�����в��������ᣮ
�ʴ�Ϊ������ȷ���������Na2CO3��Ӧ��������NaHCO3�����ų����ݣ���Na2CO3ȫ��ת��ΪNaHCO3���������NaHCO3��Ӧ����CO2���������ݣ����ԣ�û�����ݲ�����������˵�����в��������ᣮ
���������⿼�������������Ʊ����ѶȲ���ע������֪ʶ�Լ������᷽����ƽ������ۣ�

��ϰ��ϵ�д�
�����Ŀ
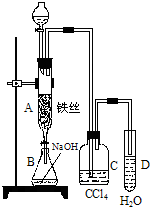 ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
 ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O