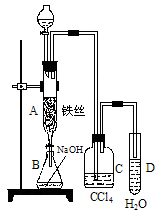
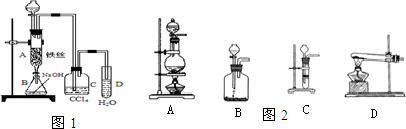
��Ŀ����
�پ�ͼ1д��A���л���Ӧ�Ļ�ѧ����ʽ
| Fe |
| Fe |
����֪�����л���Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е�������
��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����
��C��ʢ��CCl4��������
����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���
��2��ͼ2����Ȳ��ʵ�����Ʒ�
�ٷ�Ӧԭ��
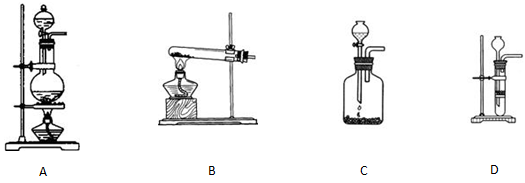
��ѡ����ʵ���ȡʵ��װ��
��ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ����
�ܴ�������Ȳ��������ɫ��ζ�����壬�õ�ʯ��ˮ��Ӧ��ȡ����Ȳ��������H2S��PH3���ж����ζ��������
| Fe |
��2��ʵ�����õ�ʯ�ͱ���ʳ��ˮ��Ӧ�Ʊ���Ȳ�����ɵ������к���H2S��PH3�����߶�������ͭ��Ӧ����������ͭ��ȥ��
| Fe |
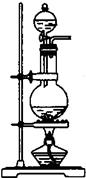
�����ڷ�Ӧ���ȣ�����Һ����ӷ�����������һ�ֺ���ɫ�����壬�ʴ�Ϊ����ӦҺ�У��к���ɫ�������A������
��A�е�����������Ʒ�Ӧ�����Խ��屽�е����ȥ����Br2+2NaOH=NaBr+NaBrO+H2O����3Br2+6NaOH=5NaBr+NaBrO3+3H2O��
�ʴ�Ϊ����ȥ�����屽�е��壻Br2+2NaOH=NaBr+NaBrO+H2O��3Br2+6NaOH=5NaBr+NaBrO3+3H2O��
�ܸ�����������ԭ�����弫���������Ȼ�̼�����廯�����ܣ�����C��ʢ��CCl4�������dz�ȥ�廯�������е����������ʴ�Ϊ����ȥ�廯�������е���������
���������ȡ����Ӧ�������廯�⣬�廯��������ˮ�����H+��Br-��ֻҪ���麬�������ӻ������Ӽ��ɣ������ӵļ��飺ȡ��Һ�μ���������Һ��������ɵ���ɫ������֤���������ӣ������ӵļ��飺�����ʹ��ɫʯ����Һ��죬��֤�����������ӣ��ʴ�Ϊ��ʯ����Һ����Һ���ɫ��
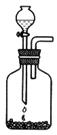
��2������ʵ������ȡ��Ȳ�����õ�ʯ��ˮ��Ӧ��ȡ�ģ�����ʽ��CaC2+2H2O��C2H2��+Ca��OH��2��
�ʴ�Ϊ��CaC2+2H2O��C2H2��+Ca��OH��2��
�����ڷ�Ӧ���ܼ��ȣ�����ѡ��A��D���Ǵ���ģ��������壬�����ó���©������ֹ�����ݳ�����C������B��ȡ���ʴ�Ϊ��B��
�����ڵ�ʯ��ˮ�ķ�Ӧ�ܾ��ң�����ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ�ļ�����ʯ��ˮ�ķ�Ӧ���ʣ��ʴ�Ϊ��������ʯ��ˮ�ķ�Ӧ���ʣ�
��H2S��PH3���ܺ�����ͭ��Һ��Ӧ����˿���������ͭ��Һ��ȥ���������壬�ʴ�Ϊ������ͭ��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д���1��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
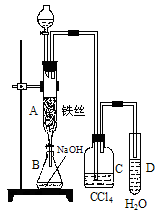
��д��A���л���Ӧ�Ļ�ѧ����ʽ
___________________________________________________
����֪�����л���Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е�������
_____________________��______________________��
�� ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_______________________________________________��д���йصĻ�ѧ����ʽ____________________________________________��
��C��ʢ��CCl4��������______________________________________��
����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���__________________��������___________________________________________��
��2����Ȳ��ʵ�����Ʒ�
�ٷ�Ӧԭ��___________________________________________________��
��ѡ����ʵ���ȡʵ��װ��_______��

 |  | ||
����
������A������������ �������� B������������������������ C�������� ������������ D
��ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ����___________________________________��
�ܴ�������Ȳ��������ɫ��ζ�����壬�õ�ʯ��ˮ��Ӧ��ȡ����Ȳ��������H2S��PH3���ж����ζ��������________________��Һ��ȥ�������塣