��Ŀ����
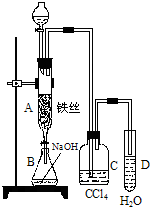 ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У���1��д��A���л���Ӧ�Ļ�ѧ����ʽ
C6H6+Br2
C6H5Br+HBr
| Fe |
C6H6+Br2
C6H5Br+HBr
| Fe |
��2����֪������Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е�������
��ӦҺ�У��к���ɫ�������A����
��ӦҺ�У��к���ɫ�������A����
��3��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����
��ȥ�����屽�е���
��ȥ�����屽�е���
��д���йصĻ�ѧ����ʽ
Br2+2NaOH=NaBr+NaBrO+H2O��3Br2+6NaOH=5NaBr+NaBrO3+3H2O
Br2+2NaOH=NaBr+NaBrO+H2O��3Br2+6NaOH=5NaBr+NaBrO3+3H2O
����4��C��ʢ��CCl4��������
��ȥ�廯�������е�������
��ȥ�廯�������е�������
����5����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���
ʯ����Һ
ʯ����Һ
����������Һ���ɫ
��Һ���ɫ
������������Һ�������۴������·���ȡ����Ӧ�����屽���廯�䣺C6H6+Br2
C6H5Br+HBr�����ڷ�Ӧ���ȣ�����Һ����ӷ������������屽���������廯��������ˮ�����H+��Br-�����������������ӻᷢ����Ӧ����AgBr�������Դ������
| Fe |
����⣺��1���ڴ����������£������ϵ���ԭ�ӱ���ԭ����ȡ���������屽��ͬʱ���廯�����ɣ��ʴ�Ϊ��C6H6+Br2
C6H5Br+HBr��
��2�����ڷ�Ӧ���ȣ�����Һ����ӷ�����������һ�ֺ���ɫ�����壬�ʴ�Ϊ����ӦҺ�У��к���ɫ�������A������
��3��A�е�����������Ʒ�Ӧ�����Խ��屽�е����ȥ����Br2+2NaOH=NaBr+NaBrO+H2O������3Br2+6NaOH=5NaBr+NaBrO3+3H2O��
�ʴ�Ϊ����ȥ�����屽�е��壻Br2+2NaOH=NaBr+NaBrO+H2O��3Br2+6NaOH=5NaBr+NaBrO3+3H2O��
��4��������������ԭ�����弫���������Ȼ�̼�����廯�����ܣ�����C��ʢ��CCl4�������dz�ȥ�廯�������е����������ʴ�Ϊ����ȥ�廯�������е���������
��5���������ȡ����Ӧ�������廯�⣬�廯��������ˮ�����H+��Br-��ֻҪ���麬�������ӻ������Ӽ��ɣ������ӵļ��飺ȡ��Һ�μ���������Һ��������ɵ���ɫ������֤���������ӣ������ӵļ��飺�����ʹ��ɫʯ����Һ��죬��֤�����������ӣ��ʴ�Ϊ��ʯ����Һ����Һ���ɫ��
| Fe |
��2�����ڷ�Ӧ���ȣ�����Һ����ӷ�����������һ�ֺ���ɫ�����壬�ʴ�Ϊ����ӦҺ�У��к���ɫ�������A������
��3��A�е�����������Ʒ�Ӧ�����Խ��屽�е����ȥ����Br2+2NaOH=NaBr+NaBrO+H2O������3Br2+6NaOH=5NaBr+NaBrO3+3H2O��
�ʴ�Ϊ����ȥ�����屽�е��壻Br2+2NaOH=NaBr+NaBrO+H2O��3Br2+6NaOH=5NaBr+NaBrO3+3H2O��
��4��������������ԭ�����弫���������Ȼ�̼�����廯�����ܣ�����C��ʢ��CCl4�������dz�ȥ�廯�������е����������ʴ�Ϊ����ȥ�廯�������е���������
��5���������ȡ����Ӧ�������廯�⣬�廯��������ˮ�����H+��Br-��ֻҪ���麬�������ӻ������Ӽ��ɣ������ӵļ��飺ȡ��Һ�μ���������Һ��������ɵ���ɫ������֤���������ӣ������ӵļ��飺�����ʹ��ɫʯ����Һ��죬��֤�����������ӣ��ʴ�Ϊ��ʯ����Һ����Һ���ɫ��
���������⿼����DZ������ʼ����ӵļ��飬������Ȼ�̼������ʱ�ӽ̲����ҳ�ԭ��--��ȡʵ�飬�������������ˣ�

��ϰ��ϵ�д�
�����Ŀ
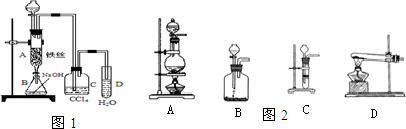
 ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�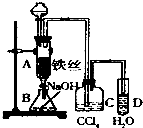 ����ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
����ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�