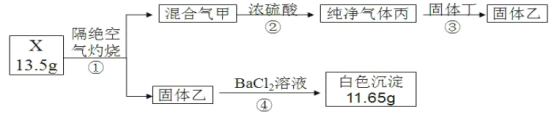
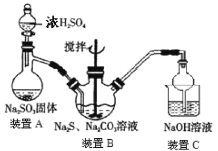
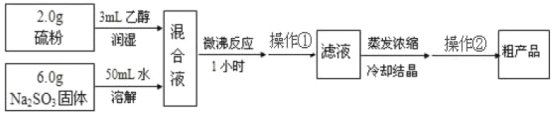
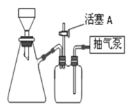
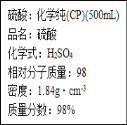
��Ŀ����
����Ŀ��ˮ������֮Դ���������ǵ�����������ء��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ���
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ______________________________��
��2��д����H2O���ӻ�Ϊ�ȵ��������_________________________����2�֣���
��3��ˮ�������ض����������õ�һ��H�����γ�ˮ�������ӣ�H3O���������ж��������̵���������������______________
A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |
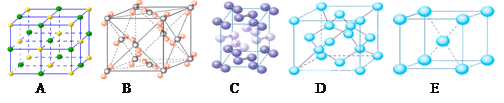
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����_________(������Ӧ�ı����д)
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����(��ͼ��ʾ)����֪������������51 kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)������������������������ ��_________kJ/mol��
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ�������ӡ���д�����ɴ������ӵ����ӷ���ʽ��__________________________________________________________��
���𰸡�1S22S22P4 H2S��NH2�� A BC 20 Cu2++4H2O��[Cu(H2O)4]2+
��������
��1�����ݺ�������Ų�������д��
��2��ԭ�����ͼ۵������ֱ���ȵ���Ϊ�ȵ�������
��3������ˮ�����Լ�ˮ���������к��еĻ�ѧ�����͡��ռ乹���жϣ�
��4�����ݱ��Ƿ��Ӿ����жϣ�
��5�����������ȣ����»���+����ж���
��6������ͭ��������ˮ�����γ���λ��������
��1����ԭ�ӵĺ˵������8�����ݹ���ԭ����֪��Χ������6�����ӣ�2s�ܼ�����2�����ӣ�2p�ܼ�����4�����ӣ�����ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ1s22s22p4��
��2��ˮ���Ӻ���3��ԭ�ӡ�8���۵��ӣ������ˮ��Ϊ�ȵ����������H2S��NH2����
��3��A��ˮ��������ԭ�Ӻ���2�����۵�����2���µ��Ӷԣ���ռ乹����V�ͣ�����ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3������ԭ�ӵ��ӻ�����û�иı䣬A����
B��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ���������״�����˸ı䣬B��ȷ��
C����ṹ��ͬ�������ʲ�ͬ�����Ļ�ѧ���ʷ����˸ı䣬C��ȷ��
D��ˮ����ΪV�ͣ�������104.5�㣬H3O+Ϊ�����ͣ����еļ��Ƿ����˸ı䣬D��ȷ����ѡA��
��4�������ڷ��Ӿ��塣�������ľ����ṹ�ص��֪��A�����Ӿ����Ȼ��Ƶľ����������������ӣ�BΪ�ɱ��ľ���ͼ��������Ϊ���ӣ����ڷ��Ӿ��壻CΪ��ľ���ͼ��������Ϊ����ӣ����Է��Ӿ��壻D��ԭ�Ӿ�����ʯ�ľ�������������ԭ�ӣ�E�ǽ��������Ƶľ������������ǽ��������Ӻ����ɵ��ӡ�������ľ���������ͬ���Ǹɱ��͵ⵥ�ʣ���ѡBC��
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����������ݽṹͼ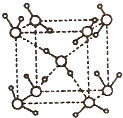 ��֪��1molˮ�к���2mol����������ȣ����»���+��������ڱ�����������51kJ/mol��ˮ���Ӽ�ķ��»�����11kJ/mol�����Ա�����������ġ����ܡ���(51kJ/mol��11kJ/mol)��2��20kJ/mol��
��֪��1molˮ�к���2mol����������ȣ����»���+��������ڱ�����������51kJ/mol��ˮ���Ӽ�ķ��»�����11kJ/mol�����Ա�����������ġ����ܡ���(51kJ/mol��11kJ/mol)��2��20kJ/mol��
��6����ˮ����ͭ����ˮ������ˮ��ͭ���ӣ�ͭ�����ṩ�չ����ˮ�����е���ԭ���ṩ�µ��Ӷԣ��γ���λ���������ɴ�������ӵ����ӷ���ʽCu2++4H2O��[Cu(H2O)4]2+��