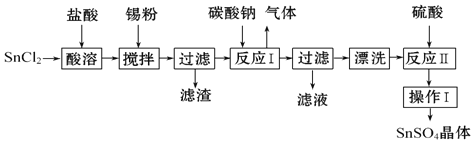
题目内容
【题目】工业上利用电解饱和食盐水来制取烧碱,所用的食盐水需两次精制。第一次精制主要是用沉淀法除去粗盐水中Ca2+、Mg2+、Fe3+、SO42-等离子,过程如下:
Ⅰ.向粗盐水中加入过量BaCl2溶液,过滤;
Ⅱ.向所得滤液中加入过量Na2CO3溶液,过滤;
Ⅲ.用盐酸调节滤液的pH,获得一次精制盐水。
(1)过程Ⅰ中除去的离子是______。
(2)表是过程Ⅰ、Ⅱ中生成的部分沉淀及其在20℃时的溶解度(g/100 gH2O):
CaSO4 | Mg2(OH)2CO3 | CaCO3 | BaSO4 | BaCO3 | Fe(OH)3 |
2.6×10-2 | 2.5×10-4 | 7.8×10-4 | 2.4×10-4 | 1.7×10-3 | 4.8×10-9 |
运用表中信息回答下列问题:
①过程Ⅱ中生成的主要沉淀除CaCO3和Mg2(OH)2CO3外还有______。
②过程Ⅰ选用的是BaCl2而不选用CaCl2,原因是______。
③除去Mg2+的离子方程式是______。
④检测Ca2+、Mg2+、Ba2+是否除尽时,只需检测Ba2+即可,原因是______。
(3)第二次精制要除去微量的I-、IO3-、NH4+、Ca2+、Mg2+,流程示意图如图:
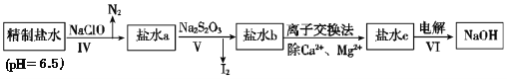
①过程Ⅳ除去的离子是______。
②盐水b中含有SO42-,Na2S2O3将IO3-还原为I2的离子方程式是______。
③在过程Ⅴ中所用的Na2S2O3俗称海波,是一种重要的化工原料。商品海波主要成分是Na2S2O3·5H2O为了测定其含Na2S2O3·5H2O的纯度,称取8.00 g样品,配制成250.0 mL溶液,取25.00 mL于锥形瓶中,滴加淀粉溶液作指示剂,再用浓度为0.0500 mol/L的碘水滴定(发生反应2S2O32-+I2=S4O62-+2I-),滴定达到终点时的现象是______。下表记录滴定结果:
滴定次数 | 滴定前读数(mL) | 滴定滴定后读数(mL) |
第一次 | 0.30 | 31.12 |
第二次 | 0.36 | 31.56 |
第三次 | 1.10 | 31.88 |
计算样品的纯度为______。
【答案】SO42- BaCO3、Fe(OH)3 BaSO4的溶解度小于CaSO4 2Mg2++2CO32-+H2O= Mg2(OH)2CO3↓+CO2↑ 碳酸钡的溶解度最大,若钡离子沉淀完全,则说明镁离子和钙离子也沉淀完全 NH4+、I- 5S2O32-+8IO3-+H2O=10SO42-+4I2+2H+ 溶液由无色变为蓝色且半分钟内不褪色 95.48%
【解析】
第一次精制:向粗盐水中加入过量BaCl2溶液,Ba2+与SO42-得到BaSO4沉淀,过滤,除去SO42-,向所得滤液中加入过量Na2CO3溶液,除去Ca2+、Mg2+、Fe3+和过量的Ba2+,得到过滤,用盐酸调节pH,除去过量的碳酸钠,得到精制盐水,
(1)根据Ba2+与SO42-的反应可得;
(2)①经过I所得滤液的杂质离子主要是:Ca2+、Mg2+、Fe3+、Ba2+,加入过量Na2CO3溶液,Ca2+、Ba2+与CO32-生成CaCO3和BaCO3,Mg2+与CO32-生成Mg2(OH)2CO3,Fe3+与CO32-发生双水解生成 Fe(OH)3;
②沉淀离子时,沉淀剂选择沉淀的越彻底越好;
③Mg2+与CO32-生成Mg2(OH)2CO3和二氧化碳,据此书写;
④Ca2+、Mg2+、Ba2+以CaCO3、Mg2(OH)2CO3、BaCO3的形式除去,根据溶解度小的沉淀完全后溶解度大的才沉淀分析;
第二次精制:向第一次精制盐水(杂质离子为I-、IO3-、NH4+、Ca2+、Mg2+)中加入NaClO,NaClO具有氧化性,将I-氧化、NH4+氧化为N2,再加入Na2S2O3,将IO3-还原为I2,分离出I2后,通过离子交换法除去Ca2+、Mg2+,电解剩余溶液得到NaOH。
(3)①根据NaClO的氧化性分析;
②Na2S2O3将IO3-还原为I2,自身被氧化为SO42-,据此书写;
③商品用碘水滴定,用淀粉溶液做指示剂,当商品反应完全时,淀粉与碘作用显色;根据反应有关系式:2S2O32-~I2,则n(Na2S2O3·5H2O)=2n(I2),根据标准液的消耗计算可得。
(1)向粗盐水中加入过量BaCl2溶液,Ba2+与SO42-得到BaSO4沉淀,过滤,除去SO42-;
(2)①经过I所得滤液的杂质离子主要是:Ca2+、Mg2+、Fe3+、Ba2+,加入过量Na2CO3溶液,Ca2+、Ba2+与CO32-生成CaCO3和BaCO3,Mg2+与CO32-生成Mg2(OH)2CO3,Fe3+与CO32-发生双水解生成 Fe(OH)3,所以过程Ⅱ中生成的主要沉淀除CaCO3和Mg2(OH)2CO3外还有BaCO3、Fe(OH)3;
②根据表可知,BaSO4的溶解度小于CaSO4,故过程Ⅰ选用的是BaCl2而不选用CaCl2;
③Mg2+与CO32-生成Mg2(OH)2CO3和CO2,离子方程式为:2Mg2++2CO32-+H2O= Mg2(OH)2CO3↓+CO2↑;
④Ca2+、Mg2+、Ba2+以CaCO3、Mg2(OH)2CO3、BaCO3的形式除去,根据溶解度小的沉淀完全后溶解度大的才开始沉淀,由表可知,碳酸钡的溶解度最大,若钡离子沉淀完全,则说明镁离子和钙离子也沉淀完全,故检测Ca2+、Mg2+、Ba2+是否除尽时,只需检测Ba2+即可;
(3)①NaClO具有氧化性,能将I-氧化,根据流程可知NH4
②Na2S2O3将IO3-还原为I2,自身被氧化为SO42-,该反应的离子方程式为:5S2O32-+8IO3-+H2O=10SO42-+4I2+2H+;
③商品用碘水滴定,用淀粉溶液作指示剂,当商品反应完全时,淀粉与碘作用显色,故当溶液由无色变为蓝色且半分钟内不褪色,说明达到滴定终点;
根据表中数据可知,第二组数据偏差较大,数据取用第一组、第三组的数据,所以用去的碘水体积为:V(碘水)=![]() mL=30.80 mL,
mL=30.80 mL,
根据反应有关系式:2S2O32-~I2,则n(Na2S2O35H2O)=2n(I2),则商品海波中Na2S2O35H2O的物质的量n(Na2S2O35H2O)=2×0.0500 mol/L×30.80×10-3L×![]() =0.0308 mol,商品海波中Na2S2O35H2O的纯度为:
=0.0308 mol,商品海波中Na2S2O35H2O的纯度为:![]() ×100%=95.48%。
×100%=95.48%。

【题目】![]() 氯丙酸
氯丙酸![]() 主要用于生产农药除草剂,还用于生产乳酸及有工业价值的低级醇酯。如图为实验室制备
主要用于生产农药除草剂,还用于生产乳酸及有工业价值的低级醇酯。如图为实验室制备![]() 氯丙酸的装置。
氯丙酸的装置。
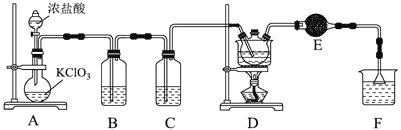
已知:相关物质的物理性质如下表所示:
物质 | 熔点 | 沸点 | 溶解性 |
| 14 | 190 | 能与水、乙醇互溶 |
丙酸 |
| 141 | 能与水、乙醇互溶 |
|
|
| 与水剧烈反应,能溶于乙醇 |
制备方法:在三颈烧瓶中放置![]() 丙酸和
丙酸和![]() 三氯化磷
三氯化磷![]() 作催化剂
作催化剂![]() ,加热至
,加热至![]() ,缓慢通入氯气,保持温度在
,缓慢通入氯气,保持温度在![]() 之间大约反应
之间大约反应![]() 。
。
回答下列问题:
![]() 装置中反应的离子方程式为_____________________________________________,当生成
装置中反应的离子方程式为_____________________________________________,当生成![]() (标准状况)时,转移电子的数目为________________。
(标准状况)时,转移电子的数目为________________。
![]() 某同学分析发现D装置有两处缺陷,分别是_____________、________________。
某同学分析发现D装置有两处缺陷,分别是_____________、________________。
![]() 设计实验提纯产品:_________________________________________________________。
设计实验提纯产品:_________________________________________________________。
![]() 测定产品纯度。
测定产品纯度。
步骤Ⅰ:称取1.20g样品![]() 杂质不含
杂质不含![]() 于烧瓶中,加入
于烧瓶中,加入![]() 氢氧化钠溶液共热,冷却至室温。加入
氢氧化钠溶液共热,冷却至室温。加入![]() 硝酸,一段时间后,将烧瓶中的溶液全部转移至
硝酸,一段时间后,将烧瓶中的溶液全部转移至![]() 容量瓶中,加水定容
容量瓶中,加水定容![]() 溶液中为乳酸和
溶液中为乳酸和![]() 。
。
步骤Ⅱ:从容量瓶中各取![]() 溶液于锥形瓶中,用
溶液于锥形瓶中,用![]() 作指示剂,用
作指示剂,用![]() 溶液分别滴定溶液中的
溶液分别滴定溶液中的![]() 已知:
已知:![]() 为砖红色沉淀、乳酸银不沉淀
为砖红色沉淀、乳酸银不沉淀![]() ,平行三次实验,所得滴定数据如表所示:
,平行三次实验,所得滴定数据如表所示:
实验序号 实验数据 | 第一次 | 第二次 | 第三次 | |
| 滴定前 | 0 |
|
|
滴定后 |
|
|
| |
![]() 加入硝酸的目的是_______________________________________。
加入硝酸的目的是_______________________________________。
![]() 步骤Ⅱ操作中,达到滴定终点的现象是___________________________________________。
步骤Ⅱ操作中,达到滴定终点的现象是___________________________________________。
![]() 样品中
样品中![]() 氯丙酸的质量分数为__________
氯丙酸的质量分数为__________![]() 保留三位有效数字
保留三位有效数字![]() 。
。
【题目】下列实验操作不正确的是
实验目的 | 实验操作 | |
A | 验证化学反应中的能量变化 | 将NO2球浸泡在冰水,热水中观察颜色变化 |
B | 证明非金属性:Cl>C>Si | 将纯碱与足量浓盐酸反应后产生的气体直接通入硅酸钠溶液中 |
C | 探究相同条件下,溶液浓度对反应速率的影响 | 在两支试管中各加入4 ml 0.01 mol/L的KMnO4 酸性溶液,再分别加入0.1 mol/L H2C2O4 溶液2 ml、0.2 mol/L H2C2O4 溶液2 mL, 分别记录溶液褪色所需时间 |
D | 除去氢氧化铁中少量的氢氧化铜 | 将过量氨水加入混合物中并充分搅拌,然后过滤、洗涤、干燥 |
A. A B. B C. C D. D