ЬтФПФкШн
ЁОЬтФПЁПАБЪЧЙЄХЉвЕЩњВњжаживЊЕФЛљДЁЮяжЪЃЌбаОПКЯГЩАБМААБЕФгІгУОпгаживЊвтвхЁЃ
ЃЈ1ЃЉвбжЊЃКN2(g)+3H2(g)=2NH3(g)ІЄH=-92kJ/molЃЌN2(g)+3H2(g)![]() 2NH3(g)ЕФЛюЛЏФмЮЊ508kJ/molЁЃдђ2NH3(g)
2NH3(g)ЕФЛюЛЏФмЮЊ508kJ/molЁЃдђ2NH3(g)![]() N2(g)+3H2(g)ЕФЛюЛЏФмЮЊ___________kJ/mol
N2(g)+3H2(g)ЕФЛюЛЏФмЮЊ___________kJ/mol
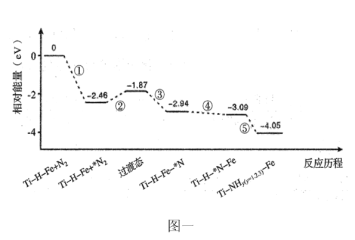
ЃЈ2ЃЉЮвЙњПЦбаШЫдБбажЦГіTi-H-FeЫЋЮТЧјДпЛЏМСЃЈTi-HЧјгыFeЧјЮТВюПЩГЌЙ§100ЁцЃЉЁЃTi-H-FeЫЋЮТЧјДпЛЏКЯГЩАБЕФЗДгІРњГЬШчЯТЭМвЛЃЌЦфжаЮќИНдкДпЛЏМСБэУцЕФЮяжжгУЁА*ЁББъзЂЁЃ
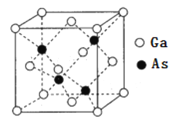
ЯТСаЫЕЗЈжае§ШЗЕФЪЧ___________ЁЃ
AЃЎЂйЮЊ![]() ЕФЖЯСбЙ§ГЬ
ЕФЖЯСбЙ§ГЬ
BЃЎЂйЂкЂлдкИпЮТЧјЗЂЩњЃЌЂмЂндкЕЭЮТЧјЗЂЩњ
CЃЎЂмЮЊNдзггЩFeЧјгђЯђTi-HЧјгђЕФДЋЕнЙ§ГЬ
DЃЎЪЙгУTi-H-FeЫЋЮТЧјДпЛЏМСЪБКЯГЩАБЗДгІзЊБфЮЊЮќШШЗДгІ
ЃЈ3ЃЉвдАБКЭCO2ЮЊдСЯКЯГЩФђЫиЕФЗДгІЮЊ2NH3(g)ЃЋCO2(g)![]() CO(NH2)2(l)ЃЋH2O(g)ЁЃЙЄвЕЩњВњЪБЃЌашвЊдСЯЦјДјгаЫЎеєЦјЃЌЭМЖўжаЧњЯпЂёЁЂЂђЁЂЂѓБэЪОдкВЛЭЌЫЎЬМБШ[
CO(NH2)2(l)ЃЋH2O(g)ЁЃЙЄвЕЩњВњЪБЃЌашвЊдСЯЦјДјгаЫЎеєЦјЃЌЭМЖўжаЧњЯпЂёЁЂЂђЁЂЂѓБэЪОдкВЛЭЌЫЎЬМБШ[![]() ]ЪБЃЌCO2ЕФЦНКтзЊЛЏТЪгыАБЬМБШ[
]ЪБЃЌCO2ЕФЦНКтзЊЛЏТЪгыАБЬМБШ[![]() ]жЎМфЕФЙиЯЕЁЃ
]жЎМфЕФЙиЯЕЁЃ
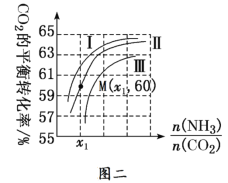
ЂйЧњЯпЂёЁЂЂђЁЂЂѓЖдгІЕФЫЎЬМБШзюДѓЕФЪЧ________ЃЌХаЖЯвРОнЪЧ________ЁЃ
ЂкВтЕУMЕуАБЦјЕФЦНКтзЊЛЏТЪЮЊ40%ЃЌдђx1ЃН______ЁЃ
ЃЈ4ЃЉНЋАБбѕЛЏЗЈжЦЯѕЫсЙ§ГЬжаАБОДпЛЏбѕЛЏКѓЕФЦјЬхЃЈИпгк800ЁцЃЉМБОчРфШДЕН100ЁцвдЯТЃЌвЛЗНУцГ§ШЅДѓСПЕФH2OЃЌЪЙNO(g)КЭO2(g)ЗЂЩњЗДгІЃЌСэвЛЗНУцЮТЖШЕЭгаРћгкЩњГЩNO2(g)ЁЃ
2NO(g)ЃЋO2(g)![]() 2NO2(g)ЕФЗДгІРњГЬЗжСНВНЃК
2NO2(g)ЕФЗДгІРњГЬЗжСНВНЃК
Ђё.2NO(g)![]() N2O2(g)(ЗДгІПьЃЌЫВМфДяЕНЦНКт)ІЄH1ЃМ0
N2O2(g)(ЗДгІПьЃЌЫВМфДяЕНЦНКт)ІЄH1ЃМ0
v1е§ЃНk1е§c2(NO) v1ФцЃНk1Фцc(N2O2)
Ђђ.N2O2(g)ЃЋO2(g)![]() 2NO2(g)(ЗДгІТ§)ІЄH2ЃМ0
2NO2(g)(ЗДгІТ§)ІЄH2ЃМ0
v2е§ЃНk2е§c(N2O2)c(O2) v2ФцЃНk2Фцc2(NO2)
Цфжаk1ЁЂk2ЪЧЫйТЪГЃЪ§ЃЌЫцЮТЖШЩЯЩ§ЖјдіДѓЁЃ
дђ:ЂйвЛЖЈЮТЖШЯТЃЌЗДгІ2NO(g)ЃЋO2(g)![]() 2NO2(g)ДяЕНЦНКтзДЬЌЃЌЧыаДГігУk1е§ЁЂk1ФцЁЂk2е§ЁЂk2ФцБэЪОЕФЦНКтГЃЪ§БэДяЪНKЃН_____ЃЌИљОнЫйТЪЗНГЬЗжЮіЃЌЩ§ИпЮТЖШИУзмЗДгІЫйТЪМѕаЁЕФдвђЪЧ__________ЁЃ
2NO2(g)ДяЕНЦНКтзДЬЌЃЌЧыаДГігУk1е§ЁЂk1ФцЁЂk2е§ЁЂk2ФцБэЪОЕФЦНКтГЃЪ§БэДяЪНKЃН_____ЃЌИљОнЫйТЪЗНГЬЗжЮіЃЌЩ§ИпЮТЖШИУзмЗДгІЫйТЪМѕаЁЕФдвђЪЧ__________ЁЃ
ЂкгЩЪЕбщЪ§ОнЕУЕНv2е§ЁЋc(O2)ЕФЙиЯЕПЩгУШчЭМБэЪОЁЃЕБxЕуЩ§ИпЕНФГвЛЮТЖШЪБЃЌЗДгІжиаТДяЕНЦНКтЃЌдђПЩФмБфЮЊЯргІЕФЕуЮЊ__(ЬюзжФИ)ЁЃ

ЁОД№АИЁП600 BC Ђѓ ЕБАБЬМБШЯрЭЌЪБЃЌЫЎЬМБШдНДѓЃЌМДдСЯЦјжаКЌЫЎеєЦјдНЖрЃЌCO2ЕФЦНКтзЊЛЏТЪдНаЁ 3 ![]() ЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйТЪБфаЁЃЌзмЗДгІЕФЫйТЪгЩНЯТ§ЕФвЛВНОіЖЈЃЌЫљвдзмЗДгІЫйТЪМѕаЁ a
ЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйТЪБфаЁЃЌзмЗДгІЕФЫйТЪгЩНЯТ§ЕФвЛВНОіЖЈЃЌЫљвдзмЗДгІЫйТЪМѕаЁ a
ЁОНтЮіЁП
(1)ЩшN2(g)+3H2(g)![]() 2NH3(g)ЕФЛюЛЏФмЮЊE1ЃЌ2NH3(g)
2NH3(g)ЕФЛюЛЏФмЮЊE1ЃЌ2NH3(g)![]() N2(g)+3H2(g)ЕФЛюЛЏФмЮЊE2ЃЌ2NH3(g)
N2(g)+3H2(g)ЕФЛюЛЏФмЮЊE2ЃЌ2NH3(g)![]() N2(g)+3H2(g)ЪЧN2(g)+3H2(g)
N2(g)+3H2(g)ЪЧN2(g)+3H2(g)![]() 2NH3(g)ЕФФцЗДгІЃЌдђE1-E2=ІЄHЃЌЫљвдЃЌ508kJ/mol -E2=-92kJ/molЃЌНтЕУЃКE2=600kJ/molЃЌЙЪД№АИЮЊЃК600ЃЛ
2NH3(g)ЕФФцЗДгІЃЌдђE1-E2=ІЄHЃЌЫљвдЃЌ508kJ/mol -E2=-92kJ/molЃЌНтЕУЃКE2=600kJ/molЃЌЙЪД№АИЮЊЃК600ЃЛ
(2)AЃЎЂйЮЊДпЛЏМСЮќИНN2ЕФЙ§ГЬЃЌAДэЮѓЃЛ
BЃЎДгЭМЩЯПЩжЊЃКЂкЙ§ГЬЮЊдкFeЧјаЮГЩЙ§ЖЩЬЌЕФЙ§ГЬЃЌЮЊЮќШШЗДгІЃЌдкИпЮТЧјЗДгІИќРћгкаЮГЩЙ§ЖЩЬЌЃЌФЧУДFeЧјЮЊИпЮТЧјЃЌTi-HЧјЮЊЕЭЮТЧјЃЌЂйЂкЂлЖМЪЧдкFeЧјЗДгІЕФЃЌМДЂйЂкЂлЖМЪЧдкИпЮТЧјЗДгІЕФЃЌЂмЂнЖМЪЧдкTi-HЧјЗДгІЕФЃЌМДЂмЂнЖМЪЧдкЕЭЮТЧјЗДгІЕФЃЌBе§ШЗЃЛ
CЃЎгЩЭМПЩжЊЃЌЂмЮЊNдзггЩFeЧјгђЯђTi-HЧјгђЕФДЋЕнЙ§ГЬЃЌCе§ШЗЃЛ
DЃЎвЛИіЗДгІЪЧЮќШШЗДгІЛЙЪЧЗХШШЗДгІгыЪЧЗёЪЙгУДпЛЏМСЁЂЪЙгУЪВУДДпЛЏМСЮоЙиЃЌжЛгыЗДгІЮяЕФЪМЬЌКЭжеЬЌгаЙиЃЌгЩКЯГЩАБЕФІЄHЃМ0ПЩжЊЃЌКЯГЩАБЮЊЗХШШЗДгІЃЌDДэЮѓЃЛ
злЩЯЫљЪіЃКBCе§ШЗЃЌADДэЮѓЃЌЙЪД№АИЮЊЃКBCЃЛ
(3)ЂйКсзјБъЃЌМДАБЬМБШ[![]() ]вЛЖЈЪБЃЌЫЎЬМБШ[
]вЛЖЈЪБЃЌЫЎЬМБШ[![]() ]діДѓЃЌдђn(H2O)діДѓЃЌc(H2O)діДѓЃЌЦНКтФцЯђвЦЖЏЃЌCO2ЕФзЊЛЏТЪгІМѕаЁЃЌЕБАБЬМБШЯрЭЌЪБЃЌЫЎЬМБШдНДѓЃЌМДдСЯЦјжаКЌЫЎеєЦјдНЖрЃЌCO2ЕФЦНКтзЊЛЏТЪдНаЁЃЌдђЂѓЮЊЫЎЬМБШзюДѓЕФЧњЯпЃЌЙЪД№АИЮЊЃКЂѓЃЛЕБАБЬМБШЯрЭЌЪБЃЌЫЎЬМБШдНДѓЃЌМДдСЯЦјжаКЌЫЎеєЦјдНЖрЃЌCO2ЕФЦНКтзЊЛЏТЪдНаЁЃЛ
]діДѓЃЌдђn(H2O)діДѓЃЌc(H2O)діДѓЃЌЦНКтФцЯђвЦЖЏЃЌCO2ЕФзЊЛЏТЪгІМѕаЁЃЌЕБАБЬМБШЯрЭЌЪБЃЌЫЎЬМБШдНДѓЃЌМДдСЯЦјжаКЌЫЎеєЦјдНЖрЃЌCO2ЕФЦНКтзЊЛЏТЪдНаЁЃЌдђЂѓЮЊЫЎЬМБШзюДѓЕФЧњЯпЃЌЙЪД№АИЮЊЃКЂѓЃЛЕБАБЬМБШЯрЭЌЪБЃЌЫЎЬМБШдНДѓЃЌМДдСЯЦјжаКЌЫЎеєЦјдНЖрЃЌCO2ЕФЦНКтзЊЛЏТЪдНаЁЃЛ
ЂкЩшCO2ЕФЦ№ЪМЮяжЪЕФСПЮЊ1ЃЌдђNH3ЕФЦ№ЪМЮяжЪЕФСПЮЊx1ЃЌMЕуЃКАБЦјЕФЦНКтзЊЛЏТЪЮЊ40%ЃЌCO2ЕФзЊЛЏТЪЮЊ60%ЃЌдђгаЃК40%x1=60%ЁС1ЁС2ЃЌНтЕУx1=3ЃЌЙЪД№АИЮЊЃК3ЃЛ
(4)ЂйЦНКтЪБЃЌv1е§ЃНv1ФцЃЌv2е§ЃНv2ФцЃЌМДk1е§c2(NO)=k1Фцc(N2O2)ЃЌk2е§=c(N2O2)ЁСc(O2)ЃНk2Фцc2(NO2)ЃЌМД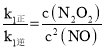 ЃЌ
ЃЌ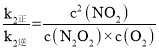 ЃЌ2NO(g)ЃЋO2(g)
ЃЌ2NO(g)ЃЋO2(g)![]() 2NO2(g)ЕФK=
2NO2(g)ЕФK=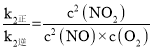 =
=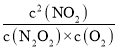 ЁС
ЁС =
=![]() ЁС
ЁС![]() =
=![]() ЁЃЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйЫйТЪБфаЁЃЌзмЗДгІЕФЫйТЪгЩНЯТ§ЕФвЛВНОіЖЈЃЌЫљвдзмЗДгІЫйТЪМѕаЁЃЌЙЪД№АИЮЊЃК
ЁЃЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйЫйТЪБфаЁЃЌзмЗДгІЕФЫйТЪгЩНЯТ§ЕФвЛВНОіЖЈЃЌЫљвдзмЗДгІЫйТЪМѕаЁЃЌЙЪД№АИЮЊЃК![]() ЃЛЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйТЪБфаЁЃЌзмЗДгІЕФЫйТЪгЩНЯТ§ЕФвЛВНОіЖЈЃЌЫљвдзмЗДгІЫйТЪМѕаЁЃЛ
ЃЛЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйТЪБфаЁЃЌзмЗДгІЕФЫйТЪгЩНЯТ§ЕФвЛВНОіЖЈЃЌЫљвдзмЗДгІЫйТЪМѕаЁЃЛ
ЂкЮТЖШЩ§ИпЦНКтN2O2(g)ЃЋO2(g)![]() 2NO2(g)ФцЯђвЦЖЏЃЌc(O2)діДѓЃЌКсзјБъБШxЕуЕФКсзјБъДѓЁЃЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйТЪБфаЁЃЌМДv2е§МѕаЁЃЌзнзјБъБШxЕуЕФзнзјБъаЁЃЌзлЩЯЫљЪіЃЌaЕуПЩФмЃЌЙЪД№АИЮЊЃКaЁЃ
2NO2(g)ФцЯђвЦЖЏЃЌc(O2)діДѓЃЌКсзјБъБШxЕуЕФКсзјБъДѓЁЃЮТЖШЩ§ИпЃЌЗДгІIФцЯђвЦЖЏВЂбИЫйДяЕНЦНКтЃЌЕМжТN2O2ХЈЖШМѕаЁЃЌЫфШЛЮТЖШЩ§Ипk2е§ЁЂk2ФцОљдіДѓЃЌЕЋЖдЗДгІЂђЕФгАЯьШѕгкN2O2ХЈЖШМѕаЁЕФгАЯьЃЌЕМжТЗДгІЂђЫйТЪБфаЁЃЌМДv2е§МѕаЁЃЌзнзјБъБШxЕуЕФзнзјБъаЁЃЌзлЩЯЫљЪіЃЌaЕуПЩФмЃЌЙЪД№АИЮЊЃКaЁЃ