��Ŀ����
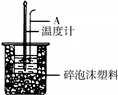 50mL 0.50mol/L�����50mL 0.55mol/L NaOH��Һ��ͼ��ʾ��װ�� �н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȣ�
50mL 0.50mol/L�����50mL 0.55mol/L NaOH��Һ��ͼ��ʾ��װ�� �н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȣ���1���ձ���������ĭ���ϵ�������
����ʵ������е�������ʧ��
����ʵ������е�������ʧ��
����2�����ձ���������Ӳֽ�壬��õ��к�����ֵ
ƫС
ƫС
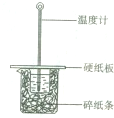
���ƫ����ƫС��������Ӱ�족����3������ͼ��ʾ������A��������
�������
�������
����ʵ������У���������¶ȼ��ϵ�����ˮ��ϴ�ɾ�ֱ�Ӳ���NaOH��Һ���¶ȣ����õġ�H��
��
-57.3KJ/mol�����������������=��������4��ʵ���и���80mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������
�����
�����
�����ȡ�������ȡ����������к���
���
���
�����ȡ�������ȡ�������5������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��
ƫС
ƫС
�������ƫ����ƫС��������Ӱ�족����6��������ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������д��ϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
��������1���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��2������Ӳֽ�壬����һ��������ɢʧ��
��3�����������Ľṹ��֪������A�ǻ��β��������������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ���ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����
��6�������к��ȵĸ����Լ��Ȼ�ѧ����ʽ����д���������
��2������Ӳֽ�壬����һ��������ɢʧ��
��3�����������Ľṹ��֪������A�ǻ��β��������������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ���ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����
��6�������к��ȵĸ����Լ��Ȼ�ѧ����ʽ����д���������
����⣺��1���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������ǣ�����ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��2�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��3������A�ǻ��β��������������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ���ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ����õ��к�����ֵ�����С������Ӧ�ȡ�H��-57.3KJ/mol��
�ʴ�Ϊ�����β�����������
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������80mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������80mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ���з�Ӧ������к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5����ˮΪ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������С��57.3kJ���ʴ�Ϊ��ƫС��
��6�������к��ȵĸ����֪���Ȼ�ѧ����ʽΪ��
H2SO4��aq��+NaOH��aq��=
Na2SO4��aq��+H2O��l����H=-57.3KJ/mol��
�ʴ�Ϊ��
H2SO4��aq��+NaOH��aq��=
Na2SO4��aq��+H2O��l����H=-57.3KJ/mol��
�ʴ�Ϊ������ʵ������е�������ʧ��
��2�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��3������A�ǻ��β��������������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ���ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ����õ��к�����ֵ�����С������Ӧ�ȡ�H��-57.3KJ/mol��
�ʴ�Ϊ�����β�����������
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������80mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������80mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ���з�Ӧ������к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5����ˮΪ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������С��57.3kJ���ʴ�Ϊ��ƫС��
��6�������к��ȵĸ����֪���Ȼ�ѧ����ʽΪ��
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��
| 1 |
| 2 |
| 1 |
| 2 |
���������⿼��ѧ���й��к��ȵIJⶨ֪ʶ�����Ը�����ѧ֪ʶ���лش�ע����к��ȸ�������⣬�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 ijͬѧ��50mL 0.50mol/L��������50mL 0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ���ǣ�������
ijͬѧ��50mL 0.50mol/L��������50mL 0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ���ǣ�������| A��ʵ�������û��������ʧ | B���ձ���������ֽ���������ǹ̶�С�ձ� | C��ͼ��ʵ��װ��ȱ�ٻ��β�������� | D���������������Ϊ60mL�������������к��Ȳ���� |
 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺ ��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺ ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ� ijͬѧ����ͼװ�����к��ȵIJⶨʵ��
ijͬѧ����ͼװ�����к��ȵIJⶨʵ��