��Ŀ����
 ��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�ٴ�ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��
����������
����������
���ڴ��ձ����粻��Ӳֽ�壬��õ��к�����ֵ��
ƫС
ƫС
���ƫ��ƫС������Ӱ�족������2������к͵ζ�����ѧ��ѧ����ʵ�飮
ijѧУ��ѧ����С����0.2000mol?L-1����ζ�δ֪Ũ�ȵ�����������Һ���Իش��������⣮
�ٵζ������У��۾�Ӧע��
��ƿ����Һ��ɫ�ı仯
��ƿ����Һ��ɫ�ı仯
����������̨�ϵ�һ�Ű�ֽ����Ŀ����
���ڹ۲���ƿ��Һ����ɫ�ı仯����С�ζ����
���ڹ۲���ƿ��Һ����ɫ�ı仯����С�ζ����
���۸����±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����
0.4000
0.4000
mol?L-1����������λ��Ч���֣�| �ζ����� | ������Һ�����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
a���۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����
ƫ��
ƫ��
��b��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����
ƫ��
ƫ��
����������1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ��
��3���ٵζ������У��۾�Ӧע����ƿ����Һ��ɫ�ı仯��
����ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ�������ǹ۲���ƿ����Һ��ɫ�ı仯���ԣ�����ʵ����
���ȷ������������Һ���������Ч�ԣ�Ȼ��������������Һ�����ƽ��ֵ��Ȼ�����c�����⣩=
���㣻
�ܸ���C�����⣩�T
������
��2�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ��
��3���ٵζ������У��۾�Ӧע����ƿ����Һ��ɫ�ı仯��
����ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ�������ǹ۲���ƿ����Һ��ɫ�ı仯���ԣ�����ʵ����
���ȷ������������Һ���������Ч�ԣ�Ȼ��������������Һ�����ƽ��ֵ��Ȼ�����c�����⣩=
| c(��)��V(��) |
| V(����) |
�ܸ���C�����⣩�T
| C(��)��V(��) |
| V(����) |
����⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β������������ʴ�Ϊ�����β�����������
��2�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��3���ٵζ������У��۾�Ӧע����ƿ����Һ��ɫ�ı仯���ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��Ϊ�˱��ڹ۲���ƿ����Һ��ɫ�ı仯������ʵ������ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ��
�ʴ�Ϊ�����ڹ۲���ƿ��Һ����ɫ�ı仯����С�ζ���
�����������Һ������ֱ�Ϊ��20.1mL��19.9mL�������ݾ���Ч�������Һ��ƽ�����Ϊ20.00mL��c�����⣩=
=
=0.4000 mol?L-1��
�ʴ�Ϊ��0.4000 mol?L-1��
��a���۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ����V������ƫ����C�����⣩�T
��������֪C�����⣩ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
b��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ������Һ�����ʵ���ƫ�����V������ƫ����C�����⣩�T
��������֪C�����⣩ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��2�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��3���ٵζ������У��۾�Ӧע����ƿ����Һ��ɫ�ı仯���ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��Ϊ�˱��ڹ۲���ƿ����Һ��ɫ�ı仯������ʵ������ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ��
�ʴ�Ϊ�����ڹ۲���ƿ��Һ����ɫ�ı仯����С�ζ���
�����������Һ������ֱ�Ϊ��20.1mL��19.9mL�������ݾ���Ч�������Һ��ƽ�����Ϊ20.00mL��c�����⣩=
| c(��)��V(��) |
| V(����) |
| 0.2000mol?L-1��20.00mL |
| 10.00mL |
�ʴ�Ϊ��0.4000 mol?L-1��
��a���۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ����V������ƫ����C�����⣩�T
| C(��)��V(��) |
| V(����) |
b��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ������Һ�����ʵ���ƫ�����V������ƫ����C�����⣩�T
| C(��)��V(��) |
| V(����) |
�ʴ�Ϊ��ƫ�ߣ�
������������Ҫ����������к͵ζ��IJ����Լ��йصĻ�ѧ���㣬�Ѷ��еȣ������к͵ζ���ԭ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ
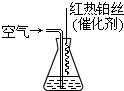 ��2013?��������ģ��������Ĥ���������Ĥ��ȽϾ��б��滯ѧ�����ȶ����ŵ㣬�ʵ�����Ĥ�����ڰ뵼�幤ҵ��Ϊ���ɵ�����Ĥ��������NH3��SiH4�����飩��һ�������·�Ӧ����600��ļ��Ȼ��������ɵ�����Ĥ��
��2013?��������ģ��������Ĥ���������Ĥ��ȽϾ��б��滯ѧ�����ȶ����ŵ㣬�ʵ�����Ĥ�����ڰ뵼�幤ҵ��Ϊ���ɵ�����Ĥ��������NH3��SiH4�����飩��һ�������·�Ӧ����600��ļ��Ȼ��������ɵ�����Ĥ��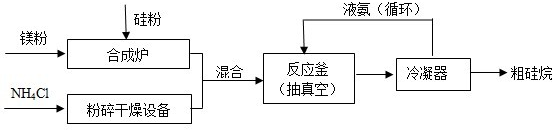

 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺