��Ŀ����
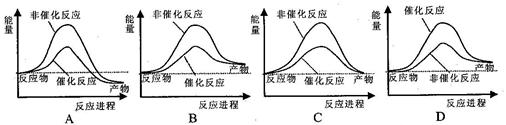
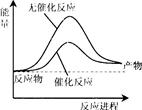
��֪��N2(g)+3H2(g) 2NH3(g)��H=" ��92" kJ/mol������������±���һ�������£������Ϊ1L���ܱ������м���1molN2��3molH2��ַ�Ӧ���ų�����Q1kJ������˵����ȷ���ǣ� ��
2NH3(g)��H=" ��92" kJ/mol������������±���һ�������£������Ϊ1L���ܱ������м���1molN2��3molH2��ַ�Ӧ���ų�����Q1kJ������˵����ȷ���ǣ� ��
| | H2(g) | N2(g) | NH3(g) |
| 1mol�����еĻ�ѧ���γ�ʱҪ�ͷų�������/kJ | 436 | 946 | a |
B��a����ֵΪ391
C��Q1����ֵΪ92
D����ͬ�����£���Ӧ����Ϊ2molN2��6molH2���ų�����Q2>2Q1
D
�������������A����ͼ��ʾ�ϳɰ����������С�����ʱ�ķ�Ӧ;���ı仯������B,a����ֵ���Ϊ�� 2a +��946+3��436��=92�����a=1173������C���÷�Ӧ�ǿ��淴Ӧ�����ܽ��е��ף��ʷų�����С��92������D,����Ӧ��Ũ��ƽ��������Ӧ�����ƶ����ų���������ԭ����2������ȷ��
���㣺���鷴Ӧ;������Ӧ�ȿ��淴Ӧ����������Ի�ѧƽ���Ӱ���֪ʶ��

��������ȼ�ϼ�С��Ⱦ���ϡ���ɫ���ˡ�������й���ȼ�ϵ�˵����ȷ����( )
| A������ȼ�����ǽ�ˮ��Ϊ�͵�����ȼ�� |
| B�������Ǿ�����ֵ�ߡ�����Ⱦ���ŵ��ȼ�� |
| C���Ҵ��DZ���������������������ȼ�� |
| D��ʯ�ͺ�ú�ǹ�������ʹ�õĿ������Ļ�ʯȼ��] |
��ȫȼ��һ����������ˮ�Ҵ����ų�������ΪQ��Ϊ��ȫ�������ɵ�CO2����ʹ֮��������Na2CO3�����ĵ�0.8mol��L NaOH��Һ500mL����ȼ��1mol�ƾ��ų���������
| A��0.2Q | B��0.1Q | C��5Q | D��10Q |
��20g Ba��OH��2��8H2O������10 g NH4Cl����һ�����С�ձ��У����ձ����ڵ���3��4��ˮ�IJ���Ƭ�ϣ��ò�����Ѹ�ٽ��衣������˵����ȷ����
| A��ʵ���в������������Ǽ��ٹ����ܽ� |
| B������Ƭ�Ͻ������С�ձ�ճ��һ��˵���÷�Ӧ�Ƿ��ȷ�Ӧ |
| C����ȡ��Ӧ�������Ķ��ٻ�Ӱ�췴Ӧ�����ȣ����Ƿ��� |
| D���÷�Ӧ�У���Ӧ���������С��������������� |
��(N2H4)�ǻ����������һ��ȼ�ϣ���ӦʱN2O4Ϊ������������N2��ˮ��������֪��
N2(g)+2O2(g)=N2O4(g) ��H="+8.7" kJ��mol-1
N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H="-534.0" kJ��mol-1
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ����
| A��2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H="-542.7" kJ��mol-1 |
| B��2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H="-1059.3" kJ��mol-1 |
C��N2H4(g)+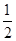 N2O4(g)= N2O4(g)=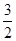 N2(g)+2H2O(g) ��H="-1076.7" kJ��mol-1 N2(g)+2H2O(g) ��H="-1076.7" kJ��mol-1 |
| D��2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H="-1076.7" kJ��mol-1 |
��֪��һ�������£�CO��ȼ����Ϊ283 kJ/mol��CH4��ȼ����Ϊ890 kJ/mol����1 mol CO��3 mol CH4��ɻ�����������������³��ȼ�գ��ͷŵ�����Ϊ
| A��2912 kJ | B��2953 kJ | C��3236 kJ | D��3867 kJ |