��Ŀ����
����Ŀ�������£�ijһԪ����HA�ĵ��볣��K=1.6��10-6����20.00mLŨ��ԼΪ0.1mol��L-1 HA��Һ����μ���0.1000mol��L-1�ı�NaOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)����ش������й����⣺
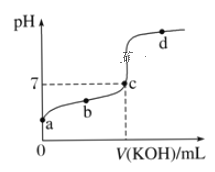
(1)a��b��c��d�ĵ���ˮ�ĵ���̶�������_______�㣬�ζ���������ѡ��__________��ָʾ�����ζ��յ���__________(����c������������c��������)��
(2)�ζ������в��ֲ������£����и�����ʹ�������ƫ�ߵ���_____(����ĸ���)��
A.�ζ�ǰ��ʽ�ζ���δ�ñ�NaOH��Һ��ϴ
B.������ˮϴ����ƿ������װ��HA��Һ����еζ�
C.�ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ�
D.�ζ�����������Һ�棬��ȡNaOH��Һ���
(3)���ظ����εζ�ʵ����������±���ʾ������ζ�����HA��Һ�����ʵ���Ũ��Ϊ_____mol/L��(����4λ��Ч����)
ʵ����� | NaOH��Һ���/mL | ����HA��Һ���/mL |
1 | 21.01 | 20.00 |
2 | 20.99 | 20.00 |
3 | 21.60 | 20.00 |
(4)a����Һ��pHԼΪ________����ʱ��Һ��H2O�������c(H��)Ϊ________��
���𰸡�c ��̪ c������ AD 0.1050 3.4 2.5��10-11mol/L
��������
(1)a��ΪHA��Һ��b����HA������KA��Һ��c����KA������HA�Ļ��Һ��d����KA��KOH�Ļ��Һ���ᡢ����Һ��������ˮ�ĵ��룬KA�ٽ�ˮ�ĵ��룻����HAΪ���ᣬǡ�÷�Ӧʱ��Һ�ʼ��ԡ�
(2)A. �ζ�ǰ��ʽ�ζ���δ�ñ�NaOH��Һ��ϴ����NaOH��ҺŨ��С���������ı�Һ���ƫ�ⶨ���ƫ�ߣ�B. ������ˮϴ����ƿ������װ��HA��Һ����еζ������´���Һ��ϡ�ͣ�ȡ��һ������Ĵ���Һ�����ʵ����ʵ������٣��ζ����������ı�Һ���ƫС���ⶨ���ƫ�ͣ�C. �ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ����������ı�Һ���ƫС���ⶨ���ƫ�ͣ�D. �ζ�����������Һ�棬��ȡNaOH��Һ����������ı�Һ���ƫ�ⶨ���ƫ�ߡ�
(3)�������������ƫ����Ҫ�����������������ı�Һ��ƽ����������ݷ�ӦHA+KOH=KA+H2O���㡣
(4)���ݵ��볣�����м���������Ũ�ȣ��ټ���pH���ټ���ˮ�������������Ũ�ȡ�
(1)a��ΪHA��Һ��b����HA������KA��Һ��c����KA������HA�Ļ��Һ��d����KA��KOH�Ļ��Һ���ᡢ����Һ��������ˮ�ĵ��룬KA�ٽ�ˮ�ĵ��룬����c��ˮ�ĵ���̶��������HAΪ���ᣬǡ�÷�Ӧʱ��Һ�ʼ��ԣ���ѡ���ڼ��Է�Χ�ڱ�ɫ��ָʾ����̪���ζ��յ�ʼ��ԣ�Ӧ����c�����ϣ��ʴ�Ϊ��c����̪��c�����ϣ�
(2)A. �ζ�ǰ��ʽ�ζ���δ�ñ�NaOH��Һ��ϴ����NaOH��ҺŨ��С���������ı�Һ���ƫ�ⶨ���ƫ�ߣ���A�������⣻B. ������ˮϴ����ƿ������װ��HA��Һ����еζ������´���Һ��ϡ�ͣ�ȡ��һ������Ĵ���Һ�����ʵ����ʵ������٣��ζ����������ı�Һ���ƫС���ⶨ���ƫ�ͣ���B���������⣻C. �ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ����������ı�Һ���ƫС���ⶨ���ƫ�ͣ���C���������⣻D. �ζ�����������Һ�棬��ȡNaOH��Һ����������ı�Һ���ƫ�ⶨ���ƫ�ߣ���D�������⣻������������Ϊ��AD��
(3)�������������ƫ����Ҫ�����������������ı�Һ��ƽ�����Ϊ��![]() �����ݷ�ӦHA+KOH=KA+H2O��֪��0.021L��0.1000 molL1=0.02L��c(HA)����ã�c(HA)=0.1050 molL1���ʴ�Ϊ��0.1050��
�����ݷ�ӦHA+KOH=KA+H2O��֪��0.021L��0.1000 molL1=0.02L��c(HA)����ã�c(HA)=0.1050 molL1���ʴ�Ϊ��0.1050��
(4)![]() ��
��![]() ��pH = 4lg4 = 42lg2 =42��0.3 =3.4����Һ�е�������Ũ��
��pH = 4lg4 = 42lg2 =42��0.3 =3.4����Һ�е�������Ũ��![]() ����Һ��������Ũ�ȵ���ˮ�������������Ũ�ȣ����ˮ�������������Ũ��Ϊc(H+)��2.5��1011molL1���ʴ�Ϊ��3.4��2.5��1011molL1��
����Һ��������Ũ�ȵ���ˮ�������������Ũ�ȣ����ˮ�������������Ũ��Ϊc(H+)��2.5��1011molL1���ʴ�Ϊ��3.4��2.5��1011molL1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������������ڸ������������ӹ����жϼ���Ӧ�����ӷ���ʽ����ȷ���ǣ� ��
ѡ�� | ���� | ������ | ���ӹ����жϼ���Ӧ�����ӷ���ʽ |
A | �μӰ�ˮ | Na+��Al3+��Cl-��NO3- | ���ܴ������棬Al3++3OH-=Al(OH)3�� |
B | ��ˮ�������H+Ũ��Ϊ1��10-12molL-1 |
| һ���ܴ�������
|
C | pH=1����Һ | Fe2+��Al3+�� | ���ܴ������棬5Fe2++ |
D | ͨ������SO2���� | K+��Na+��ClO-�� | ���ܴ������棬2ClO-+SO2+H2O=2HClO+ |
A.AB.BC.CD.D
����Ŀ��CO2��һ�����۵�̼��Դ�����ۺ����þ�����Ҫ���塣�ش��������⣺
��1��CO2���Ա�NaOH��Һ������������ҺpH=13��CO2��Ҫת��Ϊ__��д���ӷ��ţ�����������Һc��HCO3����c��CO32��=2��1����ҺpH=__���������£�H2CO3��K1=4��107��K2=5��1011��
��2��CO2��CH4�����������Ƶúϳ�����CH4��g��+CO2��g��![]() 2CO��g��+2H2��g��
2CO��g��+2H2��g��
��֪������Ӧ����صĻ�ѧ�������������£�
��ѧ�� | C��H | C=O | H��H | C |
����/kJ��mol1 | 413 | 745 | 436 | 1075 |
��÷�Ӧ����H=__���ֱ���VL�����ܱ�����A�����ݣ���B����ѹ���ݻ��ɱ䣩�У�����CH4��CO2��1mol�Ļ�����塣�������з�Ӧ��ƽ���ų������յ������϶����__������A������B������
��3����2L�ܱ������м���2molCO2��6molH2�����ʵ��Ĵ��������£�������Ӧ��CO2(g)+3H2(g)![]() CH3OH(l)+H2O(l)
CH3OH(l)+H2O(l)
������������˵���˷�Ӧ�ﵽƽ��״̬����___��
a.��������ƽ����Է����������ֲ��� b.CO2��H2������������ֲ���
c.CO2��H2��ת������� d.���������ܶȱ��ֲ���
e.1molCO2���ɵ�ͬʱ��3mol H-H������
�ڲ���״���������ȼ�ϵ�أ����������µļ״�(CH3OH)ȼ�ϵ�ظ�����Ӧʽ��__��
