��Ŀ����
ijУ��ѧ�о���ѧϰС��Ϊ̽��Cu��OH��2���ȷֽ���P���������������ʵ����̣�
��1��ȡ0.98g Cu��OH��2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ ��Cu2O��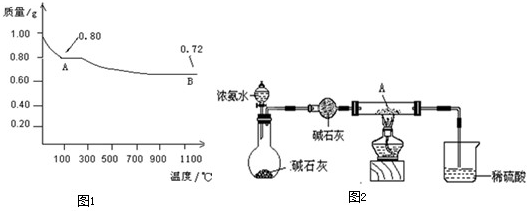
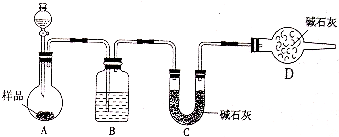
��2��Ϊ̽������A�ܷ�NH3��ԭ�����ͼ2ʵ��װ�ã��гּ�β������װ��δ������ʵ���й۲쵽A��ɺ�ɫ���ʣ�ͬʱ����һ������Ⱦ�����壬������Ļ�ѧʽΪ ��������Ϊ��װA���ʵ��Թ����ձ�֮�����һ��������װ�ã�����Ϊ�Ƿ��б�Ҫ ������С���û�С���ԭ���� ��
��3��ȡ��������B����������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�����л��к�ɫ������ڣ��÷�Ӧ�����ӷ���ʽΪ ��
��4��ͨ������ʵ������жϳ�A��B���ȶ��Դ�С�Ľ����ǣ�����ʱ ����������Һ�� ������A��B�Ļ�ѧʽ��ʾ����
��1��ȡ0.98g Cu��OH��2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ

��2��Ϊ̽������A�ܷ�NH3��ԭ�����ͼ2ʵ��װ�ã��гּ�β������װ��δ������ʵ���й۲쵽A��ɺ�ɫ���ʣ�ͬʱ����һ������Ⱦ�����壬������Ļ�ѧʽΪ
��3��ȡ��������B����������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�����л��к�ɫ������ڣ��÷�Ӧ�����ӷ���ʽΪ
��4��ͨ������ʵ������жϳ�A��B���ȶ��Դ�С�Ľ����ǣ�����ʱ
��������1������������ͭ�����������ʵ���0.01mol�����ͼ������ж����ɵIJ��
��2������ͭ�Ͱ������ȷ�Ӧ���ɺ�ɫ����Ϊͭ������Ⱦ����Ϊ���������ڷ�Ӧ���е���������������������
��3�����ݷ����ж�DΪCu2O������ ��Ӧ������ɫ��ҺΪ����ͭ����ɫ����Ϊͭ������Ԫ�ػ��ϼ۱仯����д����
��4������ͼ1��֪������������ͭ���ȶ��Դ�������ͭ�ģ����ݣ�3����֪����������Һ��������ͭ�ȶ��Խϲ
��2������ͭ�Ͱ������ȷ�Ӧ���ɺ�ɫ����Ϊͭ������Ⱦ����Ϊ���������ڷ�Ӧ���е���������������������
��3�����ݷ����ж�DΪCu2O������ ��Ӧ������ɫ��ҺΪ����ͭ����ɫ����Ϊͭ������Ԫ�ػ��ϼ۱仯����д����
��4������ͼ1��֪������������ͭ���ȶ��Դ�������ͭ�ģ����ݣ�3����֪����������Һ��������ͭ�ȶ��Խϲ
����⣺��1��0.98gCu��OH��2�������ʵ���Ϊ��
=0.01mol�����ݷֽ�ͼ������ж�100��Cʱ������ͭ�ֽ���������Ϊ0.8g������������ͭ�ֽ���������ͭ��ˮ�жϣ�Cu��OH��2=CuO+H2O������ͭĦ������Ϊ80g/mol�������ƶ�AΪCuO��
�ʴ�Ϊ��CuO��
��2��̽������ACuO�ܷ�NH3��ԭ������Ԫ�ػ��ϼۿ�֪��ͭ����۾��������ԣ������е�Ԫ�ػ��ϼ�����ͼ�-3�۾��л�ԭ�ԣ��ܷ���������ԭ��Ӧ��ʵ���й۲쵽A��ɺ�ɫ�����ж�ΪCu��ͬʱ����һ������Ⱦ��������ΪN2�����ڷ�Ӧ���е���������������������ˮ���緢���������ɽ�ˮѹ���ձ��У�
�ʴ�Ϊ��N2��û�У������ĵ�����������ˮ���緢���������ɽ�ˮѹ���ձ��У�
��3������BΪCu2O����������ϡ���ᣬ�õ���ɫ��Һ�ƶ�Ϊ����ͭ��Һ��ͬʱ�۲쵽�����л��к�ɫ�������˵������Cu�����Է�Ӧ�����ӷ���ʽΪ��Cu20+2H+=Cu2++Cu+H2O��
�ʴ�Ϊ��Cu20+2H+=Cu2++Cu+H2O��
��4����ͼ��1��֪�����ȹ���������ͭ�ȷֽ⣬˵������ͭ���ȶ��Բ������ͭ���ȶ���ǿ���ɣ�3����֪����������Һ�У�������ͭ������������ԭ��Ӧ������ͭ�ȶ��Խ�ǿ��
�ʴ�Ϊ��Cu2O�ȶ���CuO�ȶ���
| 0.98g |
| 98g/mol |
�ʴ�Ϊ��CuO��
��2��̽������ACuO�ܷ�NH3��ԭ������Ԫ�ػ��ϼۿ�֪��ͭ����۾��������ԣ������е�Ԫ�ػ��ϼ�����ͼ�-3�۾��л�ԭ�ԣ��ܷ���������ԭ��Ӧ��ʵ���й۲쵽A��ɺ�ɫ�����ж�ΪCu��ͬʱ����һ������Ⱦ��������ΪN2�����ڷ�Ӧ���е���������������������ˮ���緢���������ɽ�ˮѹ���ձ��У�
�ʴ�Ϊ��N2��û�У������ĵ�����������ˮ���緢���������ɽ�ˮѹ���ձ��У�
��3������BΪCu2O����������ϡ���ᣬ�õ���ɫ��Һ�ƶ�Ϊ����ͭ��Һ��ͬʱ�۲쵽�����л��к�ɫ�������˵������Cu�����Է�Ӧ�����ӷ���ʽΪ��Cu20+2H+=Cu2++Cu+H2O��
�ʴ�Ϊ��Cu20+2H+=Cu2++Cu+H2O��
��4����ͼ��1��֪�����ȹ���������ͭ�ȷֽ⣬˵������ͭ���ȶ��Բ������ͭ���ȶ���ǿ���ɣ�3����֪����������Һ�У�������ͭ������������ԭ��Ӧ������ͭ�ȶ��Խ�ǿ��
�ʴ�Ϊ��Cu2O�ȶ���CuO�ȶ���
���������⿼����̽��Cu��OH��2���ȷֽ������ɼ��������ʣ���Ŀ�Ѷ��еȣ���ȷԪ�ػ��ϼ۵ı仯�Ƿ����жϵĹؼ���ע�������Ϣ�ͷ�Ӧ����ķ����жϣ������ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ�����������

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ

 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������