��Ŀ����
��2011?����һģ��ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㣮ʵ���м��������ص�Ŀ����
��2��������������ͼװ�ý���ʵ�飮���ش��������⣺
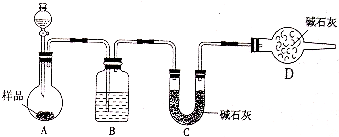
��ʵ��ǰӦ��
��ʵ���г�������Ʒ�����⣬�����
�۸��ݴ�ʵ��õ������ݣ��ⶨ����нϴ�����Ϊʵ��װ�û�����һ������ȱ�ݣ���ȱ����
��3������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������
���������жϳ����Ƿ���ȫ�ķ�����
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㣮ʵ���м��������ص�Ŀ����
��֤NaHCO3ȫ���ֽ�
��֤NaHCO3ȫ���ֽ�
����2��������������ͼװ�ý���ʵ�飮���ش��������⣺

��ʵ��ǰӦ��
���װ�õ�������
���װ�õ�������
����Һ©����Ӧ��װϡ����
ϡ����
������ᡱ��ϡ�����Ρ�����Dװ�õ����������տ����е�ˮ������������̼����ֹ����C������
���տ����е�ˮ������������̼����ֹ����C������
����ʵ���г�������Ʒ�����⣬�����
C
C
װ�ã�����ĸ��ʾ��ǰ�������ı仯���۸��ݴ�ʵ��õ������ݣ��ⶨ����нϴ�����Ϊʵ��װ�û�����һ������ȱ�ݣ���ȱ����
װ��A��B�������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ����
װ��A��B�������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ����
����3������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������
������
������
�����������жϳ����Ƿ���ȫ�ķ�����
ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ
ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ
�����������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ
55.8%
55.8%
������һλС��������������1���÷���ԭ��Ϊ�ڼ��ȵ�������Na2CO3�ܹ��ȶ����ڣ���NaHCO3���������µķֽⷴӦ2NaHCO3=Na2CO3+CO2��+H2O���Ӷ��ɸ����䷴Ӧ������CO2��ˮ����H2O����������Ĺ�����������С�մ���Ʒ�д��������������
�÷������ݼ��Ⱥ��������������⣬��ȻҪ���������أ��Ա�֤NaHCO3ȫ���ֽ⣮
��2���÷���ԭ��Ϊһ���������Ʒ��������ϡ����ֱ������·�ӦNa2CO3+H2SO4=Na2SO4+CO2��+H2O��
2 NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O����������������CO2�����������Էֱ������Ʒ��Na2CO3��NaHCO3�������Ӷ��ó�����������������÷����ؼ���Ҫ��ò�����CO2����������Ȼ��ʵ��ǰҪ���װ�õ������ԣ�Ҫ��װ���е�CO2ȫ������ʯ�������գ�Ҫ������ʯ��������CO2ǰ���������
���Ʊ�����ʵ�飬ʵ��ǰӦ�ȼ���װ�������ԣ�������лӷ��ԣ���Ӱ��ʵ������D������еļ�ʯ�����տ����е�ˮ������������̼����ֹ����C��
��ʵ�����Cװ��ǰ�������仯�ж����ɶ�����̼��������
��װ���������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ���
��3���÷���ԭ��Ϊ����Ʒ�м���BaCl2��Һ������Na2CO3����BaCl2������ӦNa2CO3+BaCl2=BaCO3��+2NaCl��
��NaHCO3��BaCl2��Ӧ���Ӷ���ߨ�����ij��������ó����������������
�����ؼ��DzⶨBaCO3��������������ȻҪ��֤������ȫ��Ҫ��ȷ���˺ã�
�ٸ��ݹ��˾�������ж�����������
���������жϳ����Ƿ���ȫ�ķ����ǣ�ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ��
����һ������Ʒ�м�������Ba��OH��2��Һ����ʱ�ֱ������·�ӦNa2CO3+Ba��OH��2=BaCO3��+2NaOH��NaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O��������BaCO3������������Ʒ������������BaCO3�����������Ϳɵó����������������
�÷������ݼ��Ⱥ��������������⣬��ȻҪ���������أ��Ա�֤NaHCO3ȫ���ֽ⣮
��2���÷���ԭ��Ϊһ���������Ʒ��������ϡ����ֱ������·�ӦNa2CO3+H2SO4=Na2SO4+CO2��+H2O��
2 NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O����������������CO2�����������Էֱ������Ʒ��Na2CO3��NaHCO3�������Ӷ��ó�����������������÷����ؼ���Ҫ��ò�����CO2����������Ȼ��ʵ��ǰҪ���װ�õ������ԣ�Ҫ��װ���е�CO2ȫ������ʯ�������գ�Ҫ������ʯ��������CO2ǰ���������
���Ʊ�����ʵ�飬ʵ��ǰӦ�ȼ���װ�������ԣ�������лӷ��ԣ���Ӱ��ʵ������D������еļ�ʯ�����տ����е�ˮ������������̼����ֹ����C��
��ʵ�����Cװ��ǰ�������仯�ж����ɶ�����̼��������
��װ���������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ���
��3���÷���ԭ��Ϊ����Ʒ�м���BaCl2��Һ������Na2CO3����BaCl2������ӦNa2CO3+BaCl2=BaCO3��+2NaCl��
��NaHCO3��BaCl2��Ӧ���Ӷ���ߨ�����ij��������ó����������������
�����ؼ��DzⶨBaCO3��������������ȻҪ��֤������ȫ��Ҫ��ȷ���˺ã�
�ٸ��ݹ��˾�������ж�����������
���������жϳ����Ƿ���ȫ�ķ����ǣ�ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ��
����һ������Ʒ�м�������Ba��OH��2��Һ����ʱ�ֱ������·�ӦNa2CO3+Ba��OH��2=BaCO3��+2NaOH��NaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O��������BaCO3������������Ʒ������������BaCO3�����������Ϳɵó����������������
����⣺��1���÷������ݼ��Ⱥ��������������⣬Ҫ���������أ��Ա�֤NaHCO3ȫ���ֽ⣮
�ʴ�Ϊ����֤NaHCO3ȫ���ֽ⣮
��2���ٸ÷�����Ҫ��ò�����CO2����������ʵ��ǰҪ���װ�õ������ԣ�������лӷ��ԣ���Ӱ��ʵ����������ѡ��ϡ���D������еļ�ʯ�����տ����е�ˮ������������̼����ֹ����C�����գ�������
�ʴ�Ϊ�����װ�õ������ԣ�ϡ������տ����е�ˮ������������̼����ֹ����C�����գ�
�ڸ�����������CO2����������Ʒ�������ֱ������Ʒ��Na2CO3��NaHCO3�������Ӷ��ó�������������������������Cװ��ǰ�������仯�ж����ɶ�����̼��������
�ʴ�Ϊ��C��
��װ��A��B�������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ���
�ʴ�Ϊ��װ��A��B�������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ���
��3���ٹ��˲����У������ձ���©����õ�������������
�ʴ�Ϊ����������
���������жϳ����Ƿ���ȫ�ķ����ǣ�ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ��
�ʴ�Ϊ��ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ��
�۷�����ӦNa2CO3+Ba��OH��2=BaCO3��+2NaOH��NaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O��
��Ʒ9.5g������ij���̼�ᱵ����Ϊ19.7g�����ʵ���Ϊ
=0.1mol������Ʒ��̼���Ƶ����ʵ���Ϊxmol��̼�����Ƶ����ʵ���Ϊymol����106x+84y=9.5��x+y=0.1�����x=0.05��y=0.05������̼���Ƶ�����Ϊ106g/mol��0.05mol=5.3g������̼������������Ϊ
��100%=55.8%��
�ʴ�Ϊ��55.8%��
�ʴ�Ϊ����֤NaHCO3ȫ���ֽ⣮
��2���ٸ÷�����Ҫ��ò�����CO2����������ʵ��ǰҪ���װ�õ������ԣ�������лӷ��ԣ���Ӱ��ʵ����������ѡ��ϡ���D������еļ�ʯ�����տ����е�ˮ������������̼����ֹ����C�����գ�������
�ʴ�Ϊ�����װ�õ������ԣ�ϡ������տ����е�ˮ������������̼����ֹ����C�����գ�
�ڸ�����������CO2����������Ʒ�������ֱ������Ʒ��Na2CO3��NaHCO3�������Ӷ��ó�������������������������Cװ��ǰ�������仯�ж����ɶ�����̼��������
�ʴ�Ϊ��C��
��װ��A��B�������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ���
�ʴ�Ϊ��װ��A��B�������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ���
��3���ٹ��˲����У������ձ���©����õ�������������
�ʴ�Ϊ����������
���������жϳ����Ƿ���ȫ�ķ����ǣ�ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ��
�ʴ�Ϊ��ȡ������Һ���ٵμ�BaCl2��Һ���������ް�ɫ�������֣�˵��������ȫ��
�۷�����ӦNa2CO3+Ba��OH��2=BaCO3��+2NaOH��NaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O��
��Ʒ9.5g������ij���̼�ᱵ����Ϊ19.7g�����ʵ���Ϊ
| 19.7g |
| 197g/mol |
| 5.3g |
| 9��5g |
�ʴ�Ϊ��55.8%��
��������ʵ��̽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������Ϊ���壬����ѧ������ʵ��ԭ����װ���������ۡ�ʵ�������������ѧ����ȣ��Ѷ��еȣ���Ŀ�漰����С�մ�ʹ���Ļ�ѧ֪ʶ�Ƕ��ģ�������һ����Ƕȵ�̽���⣮

��ϰ��ϵ�д�
�����Ŀ
 ��2011?����һģ��1983�꣬���𱤴�ѧ�����ְͿˣ�Prinzbach�����ϳɶ�л����ӣ���ͼ���ӣ�������״����ʽ���pagoda-style temple�������Ը÷���Ҳ�ͳ�Ϊpagodane�������飩���йظ÷��ӵ�˵����ȷ���ǣ�������
��2011?����һģ��1983�꣬���𱤴�ѧ�����ְͿˣ�Prinzbach�����ϳɶ�л����ӣ���ͼ���ӣ�������״����ʽ���pagoda-style temple�������Ը÷���Ҳ�ͳ�Ϊpagodane�������飩���йظ÷��ӵ�˵����ȷ���ǣ������� ��2011?����һģ����һ������Mg2+��NH4+��Al3+�����Һ�м���Na2O2���������ɳ�������������������꣩�Ĺ�ϵ��ͼ��ʾ������Һ���������ӵ����ʵ���֮��Ϊ��������
��2011?����һģ����һ������Mg2+��NH4+��Al3+�����Һ�м���Na2O2���������ɳ�������������������꣩�Ĺ�ϵ��ͼ��ʾ������Һ���������ӵ����ʵ���֮��Ϊ��������