��Ŀ����
��15�֣�50mL 0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȡ�
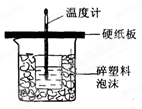
��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ������ͼ��֪��װ������������֮������1�� ����2 �� ��
��2���ձ���������������ĭ�������ǣߣߣߣߣߣߣߣߡ�
��3�����ձ����粻��Ӳֽ�壬����õķ�Ӧ����ֵ�ߣߣߡ����ƫ����ƫС��������Ӱ�족����
��4����ʵ������У���������NaOH��Һ����ʼ�¶ȵ�ƽ��ֵΪ25.2�森��Һ��Ϻ������¶�Ϊ28.6�森�Ծ�������д����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��________
�������Һ�ı�����c��4.18J/��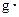 �棩�������NaOH��Һ���ܶ���Ϊ����1
�棩�������NaOH��Һ���ܶ���Ϊ����1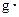
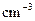 ��
��
(5)����ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬����к��ȵ���ֵ��ߣߣߣߣ��ƫ����ƫС��������Ӱ�족����

��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ������ͼ��֪��װ������������֮������1�� ����2 �� ��
��2���ձ���������������ĭ�������ǣߣߣߣߣߣߣߣߡ�
��3�����ձ����粻��Ӳֽ�壬����õķ�Ӧ����ֵ�ߣߣߡ����ƫ����ƫС��������Ӱ�족����
��4����ʵ������У���������NaOH��Һ����ʼ�¶ȵ�ƽ��ֵΪ25.2�森��Һ��Ϻ������¶�Ϊ28.6�森�Ծ�������д����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��________
�������Һ�ı�����c��4.18J/��
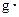 �棩�������NaOH��Һ���ܶ���Ϊ����1
�棩�������NaOH��Һ���ܶ���Ϊ����1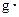
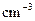 ��
��(5)����ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬����к��ȵ���ֵ��ߣߣߣߣ��ƫ����ƫС��������Ӱ�족����
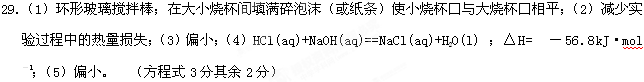
��

��ϰ��ϵ�д�
�����Ŀ
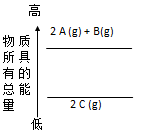
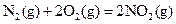 ��H=+67.7k
��H=+67.7k J��mol-1
J��mol-1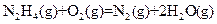 ��H=-543kJ��mol-1
��H=-543kJ��mol-1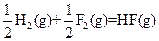 ��H=-269kJ��mol-1
��H=-269kJ��mol-1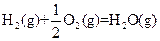 ��H=-242kJ��mol-1
��H=-242kJ��mol-1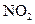 ��Ӧ���Ȼ�ѧ����ʽΪ�� ��
��Ӧ���Ȼ�ѧ����ʽΪ�� �� 2SO3����H=��196.6kJ/mol
2SO3����H=��196.6kJ/mol ��C(s) + O2(g) ="=" CO2(g)����H=" +393.5kJ/mol"
��C(s) + O2(g) ="=" CO2(g)����H=" +393.5kJ/mol"  2SO3����H=��196.6kJ/mol
2SO3����H=��196.6kJ/mol