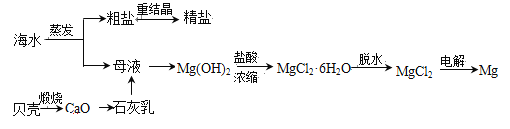
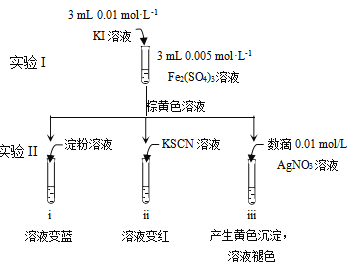
��Ŀ����
����Ŀ����1�������£���һ��2L�ĺ����ܱ������г���1 molN2��2.6 molH2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
c(NH3)/(mol��L-1) | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
��ǰ20min��ƽ�����ʦ�(H2)Ϊ__________�����¶��´˷�Ӧ��ѧƽ��ƽ�ⳣ��Ϊ_________��
������߷�Ӧ��N2��ƽ��ת���ʵ���___________��
A������N2��Ũ�� B������H2���� C���Ƴ�����NH3
D����߷�Ӧ�¶� E��������ʵĴ���
��2����ҵ�ϳɰ��ķ�ӦΪ��N2(g)+3H2(g)![]() 2NH3(g)����һ���¶��£���һ������N2��H2ͨ�뵽���Ϊ1L�ĺ����ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������___________________��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ�뵽���Ϊ1L�ĺ����ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������___________________��
A�����뺤������ѹǿ B������Ӧ���Ũ�� C��ʹ�ô��� D�������¶�
��3����֪ij��ѧ��Ӧ��ƽ�ⳣ������ʽΪK��[CO2]��[H2]/[CO]��[H2O]���ڲ�ͬ���¶��¸÷�Ӧ��ƽ�ⳣ�����±���
t�� | 700 | 800 | 830 | 1 000 | 1 200 |
K | 1.67 | 1.11 | 1.00 | 0.60 | 0.38 |
�÷�Ӧ�Ļ�ѧ������_________________________________������1 L���ܱ�������ͨ��CO2��H2��1 mol��5 min���¶����ߵ�830�棬��ʱ���CO2Ϊ0.4 mol���÷�Ӧ__________�ﵽƽ��״̬�����ǻ��
���𰸡�0.015 mol��L��1�� min��1 0.10 ��mol��L��1��-2 BC B CO(g)��H2O(g) ![]() CO2(g)��H2(g) ��
CO2(g)��H2(g) ��
��������
��1����ǰ20min�����ɰ�����Ũ����0.20mol/L������ݷ���ʽN2+3H2![]() 2NH3��֪����������Ũ����0.30mol/L����˷�Ӧ��ƽ��������(H2)Ϊ0.30mol/L��20min��0.015 mol��L��1��min��1����Ӧǰ������������Ũ�ȷֱ���0.5mol/L��1.3mol/L��ƽ��ʱ����Ũ����0.20mol/L�����ĵ�����������Ũ�ȷֱ���0.1mol/L��0.3mol/L������ƽ��ʱ������������Ũ�ȷֱ���0.4mol/L��1.0mol/L������¶��´˷�Ӧ��ѧƽ��ƽ�ⳣ��Ϊ
2NH3��֪����������Ũ����0.30mol/L����˷�Ӧ��ƽ��������(H2)Ϊ0.30mol/L��20min��0.015 mol��L��1��min��1����Ӧǰ������������Ũ�ȷֱ���0.5mol/L��1.3mol/L��ƽ��ʱ����Ũ����0.20mol/L�����ĵ�����������Ũ�ȷֱ���0.1mol/L��0.3mol/L������ƽ��ʱ������������Ũ�ȷֱ���0.4mol/L��1.0mol/L������¶��´˷�Ӧ��ѧƽ��ƽ�ⳣ��Ϊ![]() ��
��
��A������N2��Ũ��ƽ��������Ӧ������У����ή�͵�����ת���ʣ�
B������H2����������Ũ������ƽ��������Ӧ������У���ߵ�����ת���ʣ�
C���Ƴ�����NH3��������Ũ�Ƚ��ͣ�ƽ��������Ӧ������У���ߵ�����ת���ʣ�
D������Ӧ���ȣ���߷�Ӧ�¶ȣ�ƽ�����淴Ӧ������У����͵�����ת���ʣ�
E��������ʵĴ���ֻ�ܸı䷴Ӧ���ʣ����ܸı�ƽ��״̬��ת���ʲ��䡣
��ѡBC��
��2��A�����뺤������ѹǿŨ�Ȳ��䣬ƽ�ⲻ�ƶ���A�����ϣ�
B������Ӧ���Ũ�ȣ���ʹƽ��������Ӧ�����ƶ����¶Ȳ���ƽ�ⳣ�����䣬B���ϣ�
C��ʹ�ô���ƽ�ⲻ�ƶ���C�����ϣ�
D������Ӧ���ȣ������¶ȣ���ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ������D�����ϡ�
��ѡB��
��3����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����˸��ݷ�Ӧ��ƽ�ⳣ������ʽΪK��c(CO2)��c(H2)/c(CO)��c(H2O)��֪�÷�Ӧ�Ļ�ѧ����ʽ��CO(g)��H2O(g)![]() CO2(g)��H2(g)������1 L���ܱ�������ͨ��CO2��H2��1 mol��5 min���¶����ߵ�830�棬��ʱ���CO2Ϊ0.4 mol�����ݷ���ʽ��֪��������Ҳ��0.4mol������CO��ˮ��������0.4mol��ʣ��CO��ˮ��������0.6mol����ʱŨ������
CO2(g)��H2(g)������1 L���ܱ�������ͨ��CO2��H2��1 mol��5 min���¶����ߵ�830�棬��ʱ���CO2Ϊ0.4 mol�����ݷ���ʽ��֪��������Ҳ��0.4mol������CO��ˮ��������0.4mol��ʣ��CO��ˮ��������0.6mol����ʱŨ������![]() �����Ը÷�Ӧû�дﵽƽ��״̬��
�����Ը÷�Ӧû�дﵽƽ��״̬��