��Ŀ����
��8�֣�����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
2H2(g)+O2(g) ==2H2O��l�� ��H=��572kJ/mol �� ��ش��������⣺
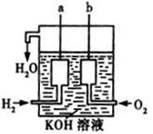 ��1�������������ܺ��������������������������������Ӧ�������ܺ͡�
��1�������������ܺ��������������������������������Ӧ�������ܺ͡�
��2����1mol������ȫȼ������ˮ��������ų������� _���������������������286 kJ��
��3����������������ɴ���ʹ����һ������װ�ã��乹���һ��������ͼ��ʾ��a��b�����缫���ɶ��̼����ɡ���װ�õĸ�����ӦʽΪ ������������5.6L����״��������ʱ�����·ת�Ƶĵ�����Ϊ ��
��ÿ��2�֣���8�֣���1���� ��2���� ��3��H2-2e-+2OH-=2H2O 6.02��1023

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�� ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol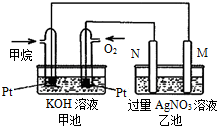 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��