��Ŀ����
 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol����1����Ӧ�������ܺ�
��2����1mol������ȫȼ�����ɶ�����̼��ˮ��������ų�������
��3����֪����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�2H2��g��+O2��g��=2H2O��l����H=-572kJ/mol������ͬ�����ļ������������ȫȼ������Һ̬ˮ�����Ƚ϶����
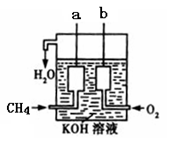
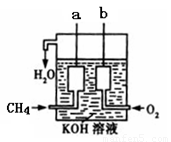
��4����ͼ��ʾ�ļ׳�װ������CH4��O2��KOH��Һ��ɵ�����ȼ�ϵ�أ����ø�װ�ÿ��Խ�
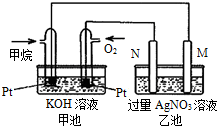
��5���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺
��6��M�缫�IJ�����
��7���ڴ˹����У��ҳ���ijһ�缫����������4.32gʱ���׳�����������������Ϊ
��������1�������Ȼ�ѧ����ʽ�С�H�ķ��ŷ�����
��2��ˮ����ת��ΪҺ̬ˮҪ�ų��������ݴ˷�����
��3�����ݷ���ʽ�����ͬ�����ļ������������ȫȼ������Һ̬ˮ�ų�������������
��4��ȼ�ϵ�ؽ���ѧ��ת��Ϊ���ܣ�
��6�����Լ���ȼ�ϵ����ͨ������һ��Ϊԭ��صĸ�����ͨ��������һ��Ϊԭ��ص��������ҳ�Ϊ���أ��ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�N����ԭ��ص�������ӦΪ���ص���������ӦΪʯī���ϣ�MΪ���ص�������Ϊ���缫�������������Һʱ��������ӦʽΪAg++e-=Ag��������ӦʽΪ4OH--4e-=O2��+2H2O��
��7�����ݵ缫��Ӧ��ϵ��ӵ�ת�Ƶ����ʵ������жϽ��м��㣮
��2��ˮ����ת��ΪҺ̬ˮҪ�ų��������ݴ˷�����
��3�����ݷ���ʽ�����ͬ�����ļ������������ȫȼ������Һ̬ˮ�ų�������������
��4��ȼ�ϵ�ؽ���ѧ��ת��Ϊ���ܣ�
��6�����Լ���ȼ�ϵ����ͨ������һ��Ϊԭ��صĸ�����ͨ��������һ��Ϊԭ��ص��������ҳ�Ϊ���أ��ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�N����ԭ��ص�������ӦΪ���ص���������ӦΪʯī���ϣ�MΪ���ص�������Ϊ���缫�������������Һʱ��������ӦʽΪAg++e-=Ag��������ӦʽΪ4OH--4e-=O2��+2H2O��
��7�����ݵ缫��Ӧ��ϵ��ӵ�ת�Ƶ����ʵ������жϽ��м��㣮
����⣺��1����֪CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol����Ӧ�Ƿ��ȷ�Ӧ�����Է�Ӧ�������ܺʹ��������������ܺͣ��ʴ�Ϊ�����ڣ�
��2��ˮ����ת��ΪҺ̬ˮҪ�ų�����������1mol������ȫȼ�����ɶ�����̼��ˮ�������ų�������С��889.6kJ���ʴ�Ϊ������
��3����֪CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��2H2��g��+O2��g��=2H2O��l����H=-572kJ/mol����1g������ȫȼ�շų�������Ϊ
��889.6kJ��1g������ȫȼ�շų�������Ϊ
��572kJ��������ͬ�����ļ������������ȫȼ������Һ̬ˮ�������ŵ������϶࣬�ʴ�Ϊ��������
��4��ԭ����ǰѻ�ѧ��ת��Ϊ���ܵ�װ�ã�����ȼ�ϵ�ؽ���ѧ��ת��Ϊ���ܣ��ʴ�Ϊ����ѧ���磻
��6�����Լ���ȼ�ϵ����ͨ������һ��Ϊԭ��صĸ����������ϼ���ʧ���ӷ���������Ӧ���缫��ӦʽΪCH4-8e-+10OH-=CO32-+7H2O��ͨ��������һ��Ϊԭ��ص��������ҳ�Ϊ���أ��ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�N����ԭ��ص�������ӦΪ���ص���������ӦΪʯī���ϣ�NΪ�������缫��Ӧʽ��4OH--4e-=O2��+2H2O��MΪ�������缫������Fe���缫��ӦʽΪAg++e-=Ag�����ҳص��ܷ�ӦʽΪ4AgNO3+2H2O
4Ag��+O2��+4HNO3��
�ʴ�Ϊ��Fe��4OH--4e-=O2��+2H2O��4AgNO3+2H2O
4Ag��+O2��+4HNO3��CH4-8e-+10OH-=CO32-+7H2O��
��7��n��Ag��=
=0.04mol������Ag++e-=Ag��֪ת�Ƶ���Ϊ0.04mol��
�׳���ͨ��������һ��Ϊ��������ӦʽΪ2O2+8H++8e-=4H2O��������n��O2��=
��0.04mol=0.01mol��
V��O2��=0.01mol��22.4L/mol=0.224L��
�����ҳ��еķ�Ӧ��֪��4Ag��O2��4HNO3������n��H+��=n��Ag��=0.04mol����c��H+��=
=0.1mol/L���ʴ�Ϊ��0.224��0.1mol/L��
��2��ˮ����ת��ΪҺ̬ˮҪ�ų�����������1mol������ȫȼ�����ɶ�����̼��ˮ�������ų�������С��889.6kJ���ʴ�Ϊ������
��3����֪CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��2H2��g��+O2��g��=2H2O��l����H=-572kJ/mol����1g������ȫȼ�շų�������Ϊ
| 1 |
| 16 |
| 1 |
| 4 |
��4��ԭ����ǰѻ�ѧ��ת��Ϊ���ܵ�װ�ã�����ȼ�ϵ�ؽ���ѧ��ת��Ϊ���ܣ��ʴ�Ϊ����ѧ���磻
��6�����Լ���ȼ�ϵ����ͨ������һ��Ϊԭ��صĸ����������ϼ���ʧ���ӷ���������Ӧ���缫��ӦʽΪCH4-8e-+10OH-=CO32-+7H2O��ͨ��������һ��Ϊԭ��ص��������ҳ�Ϊ���أ��ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�N����ԭ��ص�������ӦΪ���ص���������ӦΪʯī���ϣ�NΪ�������缫��Ӧʽ��4OH--4e-=O2��+2H2O��MΪ�������缫������Fe���缫��ӦʽΪAg++e-=Ag�����ҳص��ܷ�ӦʽΪ4AgNO3+2H2O
| ||
�ʴ�Ϊ��Fe��4OH--4e-=O2��+2H2O��4AgNO3+2H2O
| ||
��7��n��Ag��=
| 4.32g |
| 108g/mol |
�׳���ͨ��������һ��Ϊ��������ӦʽΪ2O2+8H++8e-=4H2O��������n��O2��=
| 1 |
| 4 |
V��O2��=0.01mol��22.4L/mol=0.224L��
�����ҳ��еķ�Ӧ��֪��4Ag��O2��4HNO3������n��H+��=n��Ag��=0.04mol����c��H+��=
| 0.04mol |
| 0.4L |
���������⿼���˻�ѧ��Ӧ�������ı仯��ԭ���ԭ���͵���ԭ������ȷԭ��غ͵��ص缫�Ϸ�����Ӧ�����ͼ��ɷ�������⣬�ѶȲ���ע��缫��Ӧʽ����д��������Һ��������йأ�

��ϰ��ϵ�д�
�����Ŀ