��Ŀ����
 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol��ش��������⣺
��1�������������ܺ�
��
��
������ڡ�����С�ڡ����ڡ�����Ӧ�������ܺͣ���2����2mol������ȫȼ������ˮ��������ų�������
��
��
�����������������=����572kJ����3����������������ɴ���ʹ����һ������װ�ã��乹������ͼ��ʾ��a��b�����缫���ɶ��̼����ɣ����ĸ�����Ӧ��Ϊ
H2
H2
������������5.6L����״��������ʱ�����·ת�Ƶĵ�����Ϊ6.02��1023
6.02��1023
����������1�����ݷ��ȷ�Ӧ�������������ܺ��뷴Ӧ�������ܺ͵Ĺ�ϵ��
��2�������Ȼ�ѧ����ʽ2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol���м����Լ�Һ̬ˮ���ˮ������Ҫ�������жϣ�
��3������ȼ�ϵ���и���ʧȥ���ӣ����ϼ����ߣ������õ����ӣ����ϼ۽����Լ������ĵ缫��Ӧʽ������·ת�Ƶĵ�������
��2�������Ȼ�ѧ����ʽ2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol���м����Լ�Һ̬ˮ���ˮ������Ҫ�������жϣ�
��3������ȼ�ϵ���и���ʧȥ���ӣ����ϼ����ߣ������õ����ӣ����ϼ۽����Լ������ĵ缫��Ӧʽ������·ת�Ƶĵ�������
����⣺��1������ȷ�Ӧ�������������ܣ��뷴Ӧ�������ܺͣ���2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol�Ƿ��ȷ�Ӧ���ʴ�Ϊ��С�ڣ�
��2�����Ȼ�ѧ����ʽ2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol��2mol������ȫȼ������Һ̬ˮ�ų�����572kJ����Һ̬ˮ���ˮ������Ҫ���ȣ�����2mol������ȫȼ������ˮ�����ų�����С��572kJ���ʴ�Ϊ������
��3����ȼ�ϵ�ص��ܷ�ӦΪ��2H2+O2=2H2O��ͨ�븺������H2��ͨ����������O2���缫��Ӧʽ��O2+4e-+2H2O=4OH-��
O2 +4e-+2H2O=4OH-
1mol
�ʴ�Ϊ��H2��6.02��1023 ��
��2�����Ȼ�ѧ����ʽ2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol��2mol������ȫȼ������Һ̬ˮ�ų�����572kJ����Һ̬ˮ���ˮ������Ҫ���ȣ�����2mol������ȫȼ������ˮ�����ų�����С��572kJ���ʴ�Ϊ������
��3����ȼ�ϵ�ص��ܷ�ӦΪ��2H2+O2=2H2O��ͨ�븺������H2��ͨ����������O2���缫��Ӧʽ��O2+4e-+2H2O=4OH-��
O2 +4e-+2H2O=4OH-
| 5.6L |
| 22.4L/mol |
�ʴ�Ϊ��H2��6.02��1023 ��
���������⿼��ѧ��ȼ�ϵ�ص����֪ʶ�����Ը�����ѧ�������ش��ѶȲ���

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ
 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��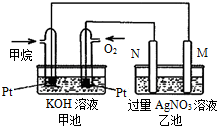 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��