��Ŀ����
����Ŀ����֪���Ը��������Һ���Խ�FeSO4��������ѧ����ʽΪ2KMnO4��10FeSO4��8H2SO4===K2SO4��2MnSO4��5Fe2(SO4)3��8H2O���ֽ�һ�����������ữ�ĸ��������Һ������������Һ��ϣ���ַ�Ӧ������������Һ�м���KI��Һ�������Һ�������ӵ����ʵ���������KI�����ʵ����ı仯��ϵ��ͼ��ʾ���������й�˵������ȷ����(����)
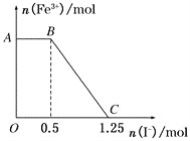
A. ͼ��AB����Ҫ�Ǹ�����غ͵⻯����Һ��Ӧ
B. ͼ��BC�η����ķ�ӦΪ2Fe3����2I��===2Fe2����I2
C. ����OC�ε����ݿ�֪��ʼ����ĸ�����ص����ʵ���Ϊ0.25 mol
D. ����OC�ε����ݿ�֪��ʼ������������������ʵ���Ϊ1 mol
���𰸡�D
��������
���������ص������Դ��������ӣ������Ӵ��ڵ⡣A��AB�������ӵ����ʵ������䣬˵����ʱ�������������ӷ���������ԭ��Ӧ����ȷ��B��BC�������ӵ����ʵ���������0��˵���öη�������������������ӵ�������ԭ��Ӧ�����ӷ���ʽ��2Fe3++2I-=" 2Fe2++" I2����ȷ��C���������Ϸ�������������������������ӣ�ʣ��������������ӣ����������������ӣ������൱�ڸ������ֱ��������ӷ���������Ӧ��������1.25molI-�������ӵ����ʵ�����1.25mol����ʧȥ���ӵ����ʵ�����1.25mol�������������������Ϊ���ʵ⣬��������ԭΪ+2�������ӣ����ݵ�ʧ�����غ㣬��������������ӷ�Ӧ�ĸ�����ص����ʵ�����1.25mol/5=0.25mol����ȷ��D����ͼ���֪���������ĵ����ӵ����ʵ�����1.25-0.5=0.75mol������2Fe3++2I-=" 2Fe2++" I2�������������ӵ����ʵ�����0.75mol�����������������ʵ�����0.75mol������ѡD��

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�


