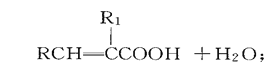��Ŀ����
����Ŀ����1��ij�¶��£������Ϊ5 L�������У�A��B��C�����������ʵ�������ʱ��仯�Ĺ�ϵ��ͼ��ʾ����÷�Ӧ�Ļ�ѧ����ʽΪ__________��2 s����A��Ũ�ȱ仯����B��Ũ�ȱ仯��ʾ��ƽ����Ӧ���ʷֱ�Ϊ________��________��
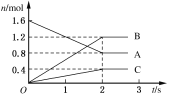
��2����0.6 mol X������0.6 mol Y��������2 L�����з�����Ӧ��2X(g)��Y(g)=nZ(g)��2W(g)��2 minĩ������0.2 mol W���������v(Z)Ũ�ȱ仯��ʾ��v(Z)��0.1 mol/(L��min)����
(1)n��_____��
(2)ǰ2 min�ڣ�v(X)��_____��
(3)2 minĩʱY��Ũ��_____��
���𰸡�2A=2B+C(���淴Ӧ) 0.08mol/(L.s) 0.12mol/(L.s) 4 0.05mol/(L.min) 0.25mol/L
��������
��1������ͼ����з������ٸ��ݻ�ѧ��Ӧ�и����ʵ����ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȵó�����ʽ��
��2�����ȼ����2min��Z�����ʵ����仯���������ʵ����뻯ѧ�����������ȼ����n��
�ڸ��ݻ�ѧ��Ӧ�����뻯ѧ�����������ȼ����ǰ2min����X��ʾ�ķ�Ӧ���ʣ�
�۸���Z�����ʵ����仯��Z��Y�ļ�������ϵ�����2min������Y�����ʵ�����Ȼ������2minʱY�����ʵ�����������c=n/V�����2minĩY��Ũ�ȡ�
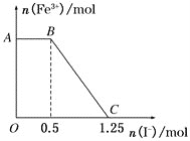
��1����ͼ����Կ���A�����ʵ�����С��B��C�����ʵ������࣬��AΪ��Ӧ�B��CΪ�������ѧ��Ӧ�и����ʵ����ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȣ�������n��A������n��B������n��C��=��1.6mol-0.8mol����1.2mol��0.4mol=2��3��1����Ӧ�Ļ�ѧ����ʽΪ2A![]() 3B+C����Ӧ��ʼ��2s����A��ʾ��ƽ����Ӧ����Ϊv=��c/��t=
3B+C����Ӧ��ʼ��2s����A��ʾ��ƽ����Ӧ����Ϊv=��c/��t=![]() =0.08mol��L-1��s-1����B��ʾ��ƽ����Ӧ����Ϊv=��c/��t=
=0.08mol��L-1��s-1����B��ʾ��ƽ����Ӧ����Ϊv=��c/��t=![]() =0.12 mol��L-1��s-1���ʴ�Ϊ��2A
=0.12 mol��L-1��s-1���ʴ�Ϊ��2A![]() 3B+C��0.08mol��L-1��s-1��0.12mol��L-1��s-1��
3B+C��0.08mol��L-1��s-1��0.12mol��L-1��s-1��
��2����ZŨ�ȱ仯����ʾ��ƽ������Ϊ0.1mol/(L��min)����Z�����ʵ����仯Ϊ��0.1mol/(L��min)��2L��2min=0.4mol���������ʵ���֮�ȵ��ڼ�����֮�ȿ���֪����n��2=0.4mol��0.2mol=2��1���ɵó���n=4���ʴ�Ϊ��4��
��Z��X�Ļ�ѧ�������ֱ�Ϊ4��2����ǰ2min����X��ʾ�ķ�Ӧ������Z��ʾ�ķ�Ӧ�����뻯ѧ�����������ȣ���v(X)=![]() v(Z)=
v(Z)=![]() ��0.1mol/(L��min)=0.05mol/(L��min)���ʴ�Ϊ��0.05mol/(L��min)��
��0.1mol/(L��min)=0.05mol/(L��min)���ʴ�Ϊ��0.05mol/(L��min)��
��2min��Z�����ʵ����仯Ϊ��0.1mol/(L��min)��2L��2min=0.4mol�����ݷ�Ӧ2X(g)��Y(g)=4Z(g)��2W(g)����֪��2minĩ�ܹ�����Y�����ʵ���Ϊ0.4mol��![]() =0.1mol����2minĩʣ��Y�����ʵ���Ϊ��0.6mol-0.1mol=0.5mol������2minĩY�����ʵ���Ũ��Ϊ��
=0.1mol����2minĩʣ��Y�����ʵ���Ϊ��0.6mol-0.1mol=0.5mol������2minĩY�����ʵ���Ũ��Ϊ��![]() =0.25mol/L���ʴ�Ϊ��0.25mol/L��
=0.25mol/L���ʴ�Ϊ��0.25mol/L��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�