��Ŀ����
17��ȫ��Һ�����ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã�ԭ����ͼ��ʾ���������Ƕ�ͭ�ĵ�Ʋۣ��������Һ�ɻ�ɫ��Ϊ��ɫ���й�˵����ȷ���ǣ�������
| A�� | ��Ӹ���ʱ�������Ϊԭ��ظ���������������Ӧ | |
| B�� | ��ӵ�Դʱ�����������ӵ�Դ��������������ԭ��Ӧ | |
| C�� | �����Ƕ�ͭ�ĵ�Ʋ�ʱ��H+��������Ҳ��ƶ� | |
| D�� | ���ʱ��ת�Ƶ�����Ϊ3.01��1023���������Һ��n��H+���ı仯��Ϊ0.5mol |
���� A���������Һ���ɻƱ�����VԪ�صĻ��ϼ���+5�۱�Ϊ+4�ۣ��õ缫�ϵõ��ӷ�����ԭ��Ӧ��
B����ӵ�Դʱ�����Һ���ɻƱ�����VԪ�صĻ��ϼ���+5�۱�Ϊ+4�ۣ�������ԭ��Ӧ��Ϊ������
C��ԭ���������������������
D�����ʱ����ת�Ƶĵ�����Ϊ3.01��1023�������ʵ���Ϊ0.5mol������ת�Ƶ��Ӻ������ӵ����ʵ���֮��Ĺ�ϵʽ�жϣ�
��� �⣺A���������Һ���ɻƱ�����VԪ�صĻ��ϼ���+5�۱�Ϊ+4�ۣ��õ缫�ϵõ��ӷ�����ԭ��Ӧ��ӦΪԭ�����������A����
B����ӵ�Դʱ�����Һ���ɻƱ�����VԪ�صĻ��ϼ���+5�۱�Ϊ+4�ۣ�������ԭ��Ӧ��Ϊ�������������������ӵ�Դ�ĸ�������B����
C�������Ƕ�ͭ�ĵ�Ʋ�ʱ�������Ϊԭ������������������������������Ҳ�������ƶ�����C����
D�����ʱ����۷����ķ�ӦΪVO2++H2O�TVO2++2H++e-����ת�Ƶ���Ϊ3.01��1023����Ϊ0.5mol����ʱ������������Ϊ1mol����ʱ�����Ӳ���������Ӧ��ͨ������Ĥ�����ƶ�ʹ����ͨ����Һ����Һ�����ӵĶ����ƶ����γɵ�����ͨ��0.5mol���ӣ��������Һ��n��H+���ı仯��Ϊ1mol-0.5mol=0.5mol����D��ȷ��
��ѡD��
���� ���⿼����ԭ��غ͵���ԭ����������ӵ�Դ������ȷ����װ�������������������ٽ�ϵ缫��Ӧʽ��������ɣ��ѵ��ǵ缫��Ӧʽ����д���Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| ���� | ���� | �Լ� | ���� | |
| A | ���� | ��ϩ | ���Ը��������Һ | ϴ�� |
| B | �Ȼ�������Һ | �Ȼ��� | �������� | ���� |
| C | �� | �� | ����NaI��Һ | ��Һ |
| D | �������� | ���� | ����̼������Һ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
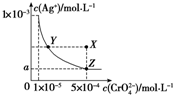
| A�� | T��ʱ����Y���Z�㣬Ag2CrO4��Ksp��� | |
| B�� | ��AgCrO4��Һ�м������K2CrO4����ʹ��Һ��Y���ΪX�� | |
| C�� | T��ʱ��Ag2CrO4��KspΪ1��10-8 | |
| D�� | ͼ��a=$\sqrt{2}$��10-4 |
| A�� | HCl�����еĹ��ۼ�����Hԭ�ӵ�1s�����Clԭ��δ�ɶԵ��ӵ�3p����γɵ�s-p�Ҽ� | |
| B�� | ʯī������̼ԭ�ӵ��ӻ��������Ϊsp2��̼ԭ�Ӽ�ֻ����sp2-sp2�Ҽ� | |
| C�� | H2O�����еĹ��ۼ�����Oԭ�ӵ�sp3�ӻ������Hԭ�ӵ�s����γɵ�s-sp3�Ҽ� | |
| D�� | N2�����еĹ��ۼ�����2��Nԭ�Ӹ�����3��p����γɵ�3��p-p�м� |
| A�� |  | B�� |  | C�� |  | D�� |  |

��ش��������⣺
��1��װ�ü�Ϊ���أ����Ե缫��������ͼʾת����ϵ��֪��AΪCl2���ѧʽ����������ӦʽΪ2H++2e-=H2����
��2��װ�ñ��ķ�Ӧ��ΪTi����װ�����������ΪTi��������װ���ڸ����������в���ì�ܣ�ԭ���ǽ���װ�ñ���Ti���е����ʽ϶࣬��װ�����г�����Ti��Ϊ����
װ������з�Ӧʱ��Ҫ�Ļ���ΪC������ĸ��ţ���
A��HCl�����Χ�� B��������Χ�� C�������Χ�� D��ˮ��
��3��װ�����з������ǹ�ҵ�ϳɼ״��ķ�Ӧ��CO��g��+2H2��g��=CH3OH��g����H��0��
�ٸ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K�����±���
| �¶�/�� | 250 | 350 |
| K | 2.041 | x |
A��0 B��0.012 C��32.081 D��100
����װ����Ϊ�ݻ��̶����ܱ���������ͬʱ��θ����ʵ�Ũ�����±���
| c��CO��/mol•L-1 | c��H2��/mol•L-1 | c��CH3OH��/mol•L-1 | |
| 0min | 0.8mol•L-1 | 1.6mol•L-1 | 0 |
| 2min | 0.6mol•L-1 | y | 0.2mol•L-1 |
| 4min | 0.3mol•L-1 | 0.6mol•L-1 | 0.5mol•L-1 |
| 6min | 0.3mol•L-1 | 0.6mol•L-1 | 0.5mol•L-1 |
Aʹ�ô��� B�����¶�C����H2��Ũ��
��4��װ�ü����Կ���ȼ�ϵ�أ���ȼ�ϵ�صĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
