��Ŀ����
ij��ѧ����С��ͨ��ʵ���о�NO2�����ʡ�
��֪��2NO2��2NaOH��NaNO3��NaNO2��H2O
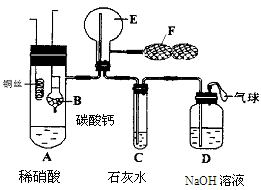
������ͼ1��ʾװ��̽��NO2�ܷ�NH3��ԭ��K1,K2Ϊֹˮ�У��г̶ֹ�װ����ȥ��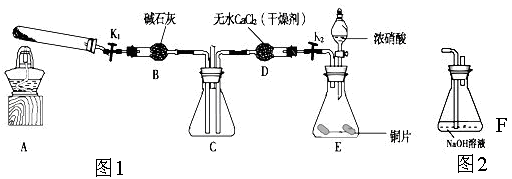
(1)Eװ������ȡNO2��Ӧ�����ӷ���ʽ�� ��
(2)��ʵ������ȡ����ʱ��ֻ��һ���Լ���������������ѡȡ ��
a��NH4HCO3 b��NH4Cl c��Ũ��ˮ
(3)��NO2�ܹ���NH3��ԭ��Ԥ�ڹ۲쵽Cװ���е������� ��
(4)ʵ����������������ã���δ�ܹ۲쵽Cװ���е�Ԥ������С��ͬѧ������ԭ������ǣ�
�٣���ԭ�Խ��������ܽ�NO2��ԭ�����ڴ������£�NO2��ת���ʼ��ͣ�
�� ��
(5)��ʵ��װ�ô���һ�����Ե�ȱ���� ��
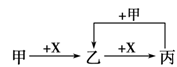
(6)Ϊ����֤NO2�ܱ�Na2O2��������С��ͬѧѡ��B��D��Eװ�ã���B�е�ҩƷ����ΪNa2O2����ѡFװ�ã���ͼ2��ʾ����������װ������ʵ�顣װ�õĺ�������˳����____��ʵ������У�Bװ���е���ɫ��ĩ��ɰ�ɫ�������飬�ð�ɫ����Ϊ��������������������ɡ��Ʋ�Bװ���з�Ӧ�Ļ�ѧ����ʽΪ________________________________��
��14�֣���1��Cu��4H����2NO3����Cu2����2NO2����2H2O��2�֣� ��2��a��2�֣�
��3��Cװ���л��������ɫ��dz������������������֣���2�֣�
��4���ڴ������£��÷�Ӧ�Ļ�ѧ��Ӧ���ʼ�����δʹ�ô�����δ���ȵȴ𰸺��������֣���2�֣�
��5��ȱ��β������װ�ã�2�֣�
��6��EDBF��FBDE��2�֣���2NO2+Na2O2��2NaNO3��2�֣�
���������������1��Ũ�������ǿ�����ԣ��ܺ�ͭ��Ӧ��������ͭ������������ˮ����Ӧ�����ӷ���ΪCu��4H����2NO3����Cu2����2NO2����2H2O��
��2��a������NH4HCO3ʱ��̼����立ֽ����ɰ�����ˮ�Ͷ�����̼����ȥˮ�Ͷ�����̼�Ϳ��Եõ���������a��ȷ��b������NH4Clʱ���Ȼ�立ֽ����ɰ������Ȼ��⣬���¶Ƚ��ͺ������Ȼ����ַ�Ӧ�����Ȼ�泥����Եò�����������b����ȷ��c��Ũ��ˮ��Һ�壬�Թ��е�ҩƷ�ǹ��壬���Բ�����Ҫ��c����ȷ����ѡa��
��3�����������Ǻ���ɫ���壬����ܱ�������ԭ��������ɫ���嵪������Cװ���л��������ɫ��dz��
��4��δ�ܹ۲쵽Cװ���е�Ԥ��������˵����Ӧδ�ܷ��������ķ�Ӧ����٣�������δ�ܹ۲쵽Cװ���е�Ԥ���������ԭ���ǣ��ڴ������£��÷�Ӧ�Ļ�ѧ��Ӧ���ʼ�����δʹ�ô�����δ���Ȼ�NO2��ת���ʼ��͵��·�Ӧ����仯�����ԡ�
��5�������������ж����壬�������д̼�����ζ�����壬���Զ�����ֱ���ſգ�Ҫ����β�����������ſա�
��6����֤�������ƺͶ���������Ӧ������Ҫ��Eװ����ȡ������������Ϊ��ȡ�Ķ������������к���ˮ������ˮ�ܺ������Ʒ�Ӧ����ɸ��ţ�����Ҫ��Dװ�ó�ȥˮ������Ȼ��ϴ����Ķ�������ͨ��Bװ�ã�δ��Ӧ�Ķ��������ж�����ֱ���ſգ��������Ҫ����β��������ѡ��ͼ2װ�ô���β��������װ�õĺ�������˳����EDBF��FBDE ��Bװ���е���ɫ��ĩ��ɰ�ɫ�������飬�ð�ɫ����Ϊ��������������������ɣ�����������������������������е�Ԫ��ʧ���ӻ��ϼ����߶�����ԭ�������Ե�Ԫ��ֻ��ת��Ϊ+5�ۣ����������Ǵ���������������ƣ���˷�Ӧ�Ļ�ѧ����ʽΪ2NO2+Na2O2��2NaNO3��
���㣺����������ͭ�ķ�Ӧ�������Ʊ���NO2�����ʣ�ʵ�鷽����������ۣ�β�������Լ�������ԭ��Ӧ���й��жϺ�Ӧ�õ�


 B
B C
C D
D F
F