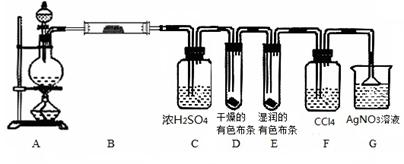
��Ŀ����
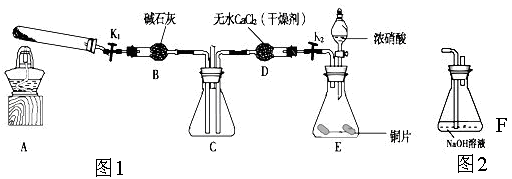
Ϊ��֤��ͭ��ϡ���ᷴӦ����NO��ijУѧ��ʵ��С�������һ��ʵ�飬��װ����ͼ��ʾ������װ�ú̶�װ�þ�����ȥ����BΪһ���ý���˿�̶��ĸ���ܣ���װ��״̼��ƹ��壻EΪһ���յ�������ƿ��F�����ڹ��������˫��������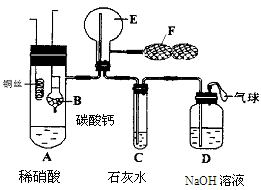
��1�� ʵ��ʱ���Ƚ�Bװ�����ƣ�ʹ̼�����ϡ����Ӵ��������壬��C��������ɫ����ʱ�����̽�Bװ�����ᣬʹ֮��ϡ������롣����ѧ����ƴ˲�������Ŀ��Ϊ ��
��2����A��ͭ˿����ϡ�����У���װ��A���ȣ���װ��A�в�����ɫ���壬�䷴Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��E�п�ʼʱ����dz����ɫ���壬��F��E�й�������ɹ۲쵽��ƿE��������ɫ����������������ԭ���� ��
��4��һ��ʱ���C�а�ɫ�����ܽ⣬��ԭ����________________________ __��
��5��װ��D��������_________ ___________________________________��
��10�֣���1����̼�����ϡ���ᷴӦ�����Ķ�����̼�ų�װ���еĿ���
��2��3Cu + 8HNO3(ϡ)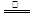 3Cu(NO3)2 + 2NO��+ 4H2O
3Cu(NO3)2 + 2NO��+ 4H2O
��3��CO2�ܶȱȿ�����CO2����E�в�δ��ƿ�п�����ȫ�ž�����ʹ������NO������������NO2��������dz����ɫ������F�й������ʱ������NO��������NO2���ʺ���ɫ����
��4��NO2����Һ�����ɵ�HNO3��CaCO3��Ӧ��ʹ�����ܽ�
��5������β������ֹ��Ⱦ����
���������������1������ϡ����Ļ�ԭ������NO����NO���ױ�������������NO2����װ���к��п���������ʵ��ʱ���Ƚ�Bװ�����ƣ�ʹ̼�����ϡ����Ӵ��������壬��C��������ɫ����ʱ�����̽�Bװ�����ᣬʹ֮��ϡ������롣����ѧ����ƴ˲�������Ŀ��Ϊ��̼�����ϡ���ᷴӦ�����Ķ�����̼�ų�װ���еĿ�������ֹ����NO�ļ��顣
��2�����������������ͭ��Ӧ�Ļ�ѧ����ʽ��3Cu + 8HNO3(ϡ)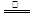 3Cu(NO3)2 + 2NO��+ 4H2O��
3Cu(NO3)2 + 2NO��+ 4H2O��
��3������CO2�ܶȱȿ�����CO2����E�в�δ��ƿ�п�����ȫ�ž�����ʹ������NO������������NO2��������dz����ɫ������F�й������ʱ������NO��������NO2���ʺ���ɫ���
��4������NO2����Һ����ˮ��Ӧ���ɵ�HNO3��CaCO3��Ӧ����ʹ�����ܽ⡣
��5��NO��NO2�����ж��ģ����ڴ�����Ⱦ���Ҫβ�����������װ��D������������β������ֹ��Ⱦ������
���㣺����ͭϡ���ᷴӦ���й�ʵ��̽��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ���������У������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ�����������ѧ���淶�Ͻ���ʵ����������ʹ���˼ά����������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�ij�����ĩ���п��ܺ���K2CO3��KNO3��NaNO2��K2SO3��Na2SO4��FeO��Fe2O3�е������֣�ijͬѧΪȷ���ù����ĩ�ijɷ֣�ȡ��������ʵ�飬ʵ����̼��������£� 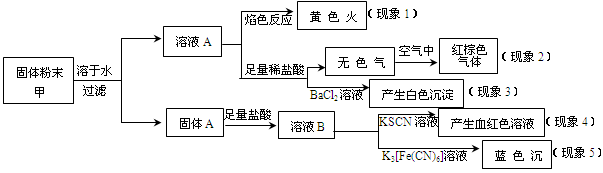
��ͬѧ�ó��Ľ�����ȷ����
| A����������1���Ƴ��ù����ĩ�к�����Ԫ�أ���������Ԫ�� |
| B����������2���Ƴ��ù����ĩ��һ������NaNO2 |
| C����������3���Ƴ��ù����ĩ��һ������Na2SO4 |
| D����������4������5���Ƴ��ù����ĩ��һ������FeO��Fe2O3 |
���� ������˵������ȷ��
������˵������ȷ��
��Xһ���ǵ��ʣ�
�ڷ�Ӧ��һ���ǻ��Ϸ�Ӧ��
����ǿ��ΪHNO3��������һ�������������
����ǿ��ΪNaOH��������һ����Na2O��
| A���٢� | B���ڢ� | C���ڢۢ� | D��ȫ�� |
��֪�ס��ҡ�����X��������ѧ��ѧ�г��������ʣ���ת����ϵ����ͼ�����X��������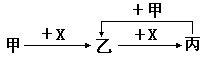
| A����ΪC��X��O2 | B����ΪSO2��X��NaOH��Һ |
| C����ΪCl2��XΪFe | D����ΪAl��XΪNaOH��Һ |
�±���������֮��ͨ��һ����Ӧ������ʵ����ͼ��ʾ��ת����ϵ����(����)��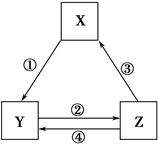
| ѡ�� | X | Y | Z | ��ͷ���������ֵķ�Ӧ���� |
| A | CaO | Ca(OH)2 | CaCO3 | �ٳ�����ˮ |
| B | AlCl3 | NaAlO2 | Al(OH)3 | ��ͨ��CO2 |
| C | Fe2O3 | FeCl3 | Fe(OH)3 | �ܼ������� |
| D | Cl2 | Ca(ClO)2 | HClO | �ۼ�Ũ���� |