��Ŀ����
����Ŀ��ij�л��� A��C4H6O5���㷺����������ˮ���ڣ�����ƻ�������ѡ����ϡ�ɽ���Ϊ�ࡣ�û���������������ʣ�
�� ��25��ʱ�����볣��K1��3.99��10-4��K2��5.5��10-6
��A��RCOOH����ROH��![]() ����ζ�IJ���
����ζ�IJ���
��1molA![]() ��������l.5mol����
��������l.5mol����
��A��һ���¶��µ���ˮ������ǻ�״������ɺ���ˮ�����ӳɷ�Ӧ
��A����5�ֲ�ͬ��ѧ��������
�Իش�
��l������������Ϣ����A�Ľṹ���������ж���__________����ѡ�۷֣�
��a���϶���̼̼˫�� ��b���������Ȼ�
��c���϶����ǻ� ��d���У�COOR������
��2���л���A�Ľṹ��ʽΪ_____________________________________________________
��3��A��һ���¶��µ���ˮ�������ˮ��Ӧ�Ļ�ѧ����ʽ��_________________________________________________________
��4��A��һ��ͬ����ͬ���칹����(������)_____________________________
���𰸡� b��c HOOCCHOHCH2COOH HOOCCH=CHCOOH + Br2 �� HOOCCHBrCHBrCOOH HOCH2CH(COOH)2
�����������⿼���л��ƶϣ���Ϲ����ŵ����ʣ�����Ϣ���֣�������������ƽ�ⳣ����˵�����л������ڶ�Ԫ���ᣬ������2���Ȼ������ܸ��Ȼ����ǻ�����������˵�����л����к����Ȼ����ǻ�����1molA���������Ʒ�Ӧ����1.5molH2��˵��1molA�к���1mol�ǻ���2mol�Ȼ�����A�ܷ�����ȥ��Ӧ��˵���ǻ�����̼ԭ�����ڵ�̼�����⣻��A�������ֲ�ͬ��ѧ�������⣻�����������Ƴ�AΪHOOC��CH(OH)��CH2��COOH����1������������������ѡ��bc��ȷ����2��A�Ľṹ��ʽΪ��HOOC��CH(OH)��CH2��COOH����3��A��һ���¶��·�����ˮ�������������ˮ��Ӧ��˵��A��������ȥ��Ӧ������ȥ��Ӧ��IJ�����HOOC��CH=CH��COOH������ˮ�����ӳɷ�Ӧ���䷴Ӧ����ʽΪ��HOOCCH=CHCOOH + Br2 �� HOOCCHBrCHBrCOOH ����4������������ͬ���칹����HOCH2CH(COOH)2��

 ��ս�п�����ϵ�д�
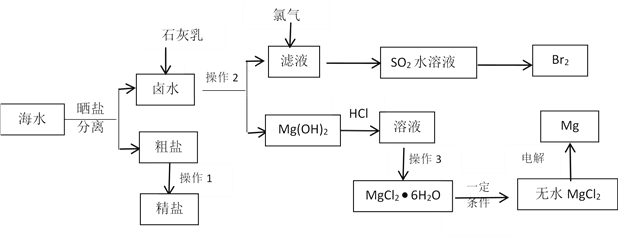
��ս�п�����ϵ�д�����Ŀ����ҵ����O2��HClת��ΪCl2���ɼ�����Ⱦ��
��1���ڴ�ͳ���������������¿�ʵ�ָ�ת����4HCl+O2![]() 2H2O+2Cl2��������ת�Ƶķ������Ŀ__________���÷�Ӧ���������ԣ�O2______Cl2��ѡ�>������<����=������������3.55g Cl2��ת�Ƶ��ӵ����ʵ���Ϊ��___________��
2H2O+2Cl2��������ת�Ƶķ������Ŀ__________���÷�Ӧ���������ԣ�O2______Cl2��ѡ�>������<����=������������3.55g Cl2��ת�Ƶ��ӵ����ʵ���Ϊ��___________��
��֪������RuO2����������HClת��ΪCl2���ܷ�Ӧ���и��õĴ����ԡ�
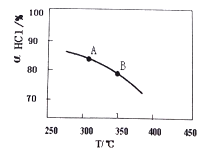
��2������RuO2������ʹ�ö���ƽ�ⳣ����Ӱ���ǣ�__________��ѡ�����ˡ�������С�ˡ�����Ӱ�족���� ʵ������һ��ѹǿ�£��÷�Ӧ��HClƽ��ת�������¶ȱ仯�Ħ�HCl��T��������ͼ���÷�Ӧ��__________��ѡ����ȷ�Ӧ���������ȷ�Ӧ����û�����Եķ��Ȼ����ȡ�����A��B�����ƽ�ⳣ��K(A)��K(B)�нϴ����____________________��
��3���������������䣬ѹ�����ʹѹǿ�������HCl��__________��ѡ���������С�����䡱��������Ҫ˵�����ɣ�___________________��
��4��һ�������£�2���ܱ������У���÷�Ӧ������n(Cl2)���������£�
��min�� | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n(Cl2)/10-3mol | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
����2.0~6.0min����HCl�ı仯��ʾ�ķ�Ӧ����_________________��
��5��Cl2��;�㷺��д����Cl2�Ʊ�Ư�۾��Ļ�ѧ����ʽ��______________��
����Ư�۾�����Ч�ɷ��ǣ�_________________�����������ƣ������ڿ������ױ��ʵ���Ҫԭ���ǣ�_______________________��