��Ŀ����
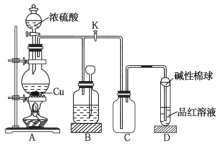
����Ŀ��ͭ��Ũ��������ͼ��ʾװ���з�����Ӧ��ʵ���й۲쵽������Ϊ![]() �Թ�����Һ��Ϊ��ɫ���Թܵײ����ֺ�ɫ�ͻҰ�ɫ������
�Թ�����Һ��Ϊ��ɫ���Թܵײ����ֺ�ɫ�ͻҰ�ɫ������![]() �Թ����ȳ������ݣ���ʱ����Ʒ����Һ�ޱ仯��֮����Һ�ĺ�ɫ��dzֱ����ȥ���Իش��������⣺
�Թ����ȳ������ݣ���ʱ����Ʒ����Һ�ޱ仯��֮����Һ�ĺ�ɫ��dzֱ����ȥ���Իش��������⣺
(1)![]() �Թ��з�����Ӧ�Ļ�ѧ����ʽΪ______��
�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ______��
(2)![]() �Թ����ȳ��ֵ�����Ϊ______�������ƣ���
�Թ����ȳ��ֵ�����Ϊ______�������ƣ���
(3)ʵ����Ϻ�ȡ��![]() �Թܣ�______������Һ______����֤��ʹƷ����Һ��ɫ������Ϊ
�Թܣ�______������Һ______����֤��ʹƷ����Һ��ɫ������Ϊ![]() ��
��
(4)![]() �Թ��е�������______��
�Թ��е�������______��
(5)ʪ�����ŵ�������______��
(6)ʵ����Ϻ���Һ��ȴ��ȡ![]() �Թ����ϲ���Һ����ˮ�У�������Һ�¶����ߡ��Խ�����Һ�¶����ߵ�ԭ��____________��
�Թ����ϲ���Һ����ˮ�У�������Һ�¶����ߡ��Խ�����Һ�¶����ߵ�ԭ��____________��
(7)����Ӧ��ij�������ˮ�У���ɫ�������ܽ⣬�Ұ�ɫ�������ܽ⣬��Һ��Ϊ��ɫ����Ұ�ɫ��������Ҫ�ɷ�Ϊ____________��
(8)��ɫ��������Ϊ![]() ��
��![]() ��
��![]() ��������ѧ֪ʶ�����ʵ����֤��ɫ�������Ƿ���
��������ѧ֪ʶ�����ʵ����֤��ɫ�������Ƿ���![]() ����֪
����֪![]() ��
��![]() ��Ϊ��ɫ���壬������ˮ��ϡ���ᣩ��____________��
��Ϊ��ɫ���壬������ˮ��ϡ���ᣩ��____________��
���𰸡�![]() ��Ũ��
��Ũ��![]() ���� ���� �ָ���ɫ ��ɫʯ����Һ��� ����
���� ���� �ָ���ɫ ��ɫʯ����Һ��� ����![]() β������ֹ��Ⱦ���� ��Ӧ����Ũ�����������ˮϡ��ʱ�ų��������ȣ�ʹ��Һ�¶�����
β������ֹ��Ⱦ���� ��Ӧ����Ũ�����������ˮϡ��ʱ�ų��������ȣ�ʹ��Һ�¶����� ![]() ȡ������ɫ��������ϡ���ᣬ����Һ���������ɫ�����к���
ȡ������ɫ��������ϡ���ᣬ����Һ���������ɫ�����к���![]() ������Һ�����������ɫ�����в���
������Һ�����������ɫ�����в���![]()
��������
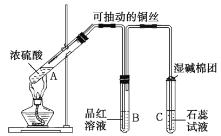
ͭ��Ũ���ᷴӦ����CuSO4��SO2���ɳ鶯��ͭ˿�����Կ��Ʒ�Ӧ�ķ�����ֹͣ��SO2��ʹƷ����Һ��ɫ��SO2����ˮ����H2SO3��ʹʯ����Һ�ʺ�ɫ��պ�м���Һ����������β�����ա�
(1)A�Թ��У�ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ����ѧ����ʽΪ![]() (Ũ)
(Ũ) ![]() CuSO4��SO2����2H2O��
CuSO4��SO2����2H2O��
(2)װ�����п������ڣ���B�Թ����ȳ��ֵ�����Ϊ������
(3)�����������Ư���ԣ�����ijЩ��ɫ�������ɵ���ɫ���ʲ��ȶ�������Ⱥ�Ʒ����Һ�ָ���ɫ����ʵ����Ϻ�ȡ��B�Թܣ����ȣ�����Һ�ָ���ɫ����֤��ʹƷ����Һ��ɫ������Ϊ![]() ��
��
(4)����������ˮ��Ӧ���������ᣬ��ʹ��ɫʯ����Һ��죻
(5)�������������ж����ܱ���Һ���գ���ʪ�����ŵ�����������![]() β������ֹ��Ⱦ������
β������ֹ��Ⱦ������
(6)��Ӧ����Ũ�����������ȡ�ϲ���Һ��ˮϡ��ʱ���ų��������ȣ�ʹ��Һ�¶����ߣ�
(7)���������Ϣ�ƶϣ��Ұ�ɫ��������Ҫ�ɷ�ΪCuSO4��
(8)![]() ��ϡ���ᷴӦ��������ͭ��ˮ�����������Ϣ֪
��ϡ���ᷴӦ��������ͭ��ˮ�����������Ϣ֪![]() ��
��![]() ��ϡ�����Ӧ������֤��ɫ�������Ƿ���
��ϡ�����Ӧ������֤��ɫ�������Ƿ���![]() ��ʵ�鷽��Ϊ��ȡ������ɫ��������ϡ���ᣬ����Һ���������ɫ�����к���
��ʵ�鷽��Ϊ��ȡ������ɫ��������ϡ���ᣬ����Һ���������ɫ�����к���![]() ������Һ�����������ɫ�����в���
������Һ�����������ɫ�����в���![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
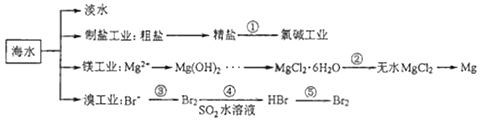
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij���̿����Ҫ�ɷ�ΪMnCO3��������������FeCO3��CaCO3��MgCO3��Al2O3�Լ�һЩ����Ӧ�IJ��������ʣ���ҵ�������̿�Ϊԭ���Ʊ��ߴ���̼���̵�������ͼ��ʾ��

��֪��MnCO3+2NH4Cl![]() MnCl2+CO2��+2NH3��+H2O
MnCl2+CO2��+2NH3��+H2O
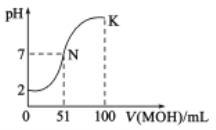
��ؽ�������[c0(Xn+)=0.1mol��L-1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Ca2+ | Mg2+ | Al3+ | Fe3+ |
��ʼ����ʱ��pH | 8.1 | 6.3 | 10.6 | 8.9 | 3.4 | 1.5 |
��ȫ����ʱ��pH | 10.1 | 8.3 | 13.1 | 10.9 | 4.7 | 2.8 |
��1������¯�����ɵ�����A�ijɷ���__���ѧʽ����
��2������A��������ȡ�������ȡ������ͼ��ʾ��
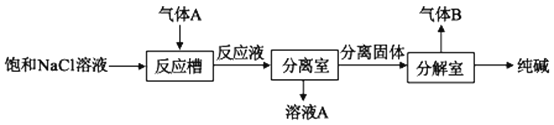
��Ӧ�����г����������ó�����__���ѧʽ�������ɸó��������ӷ�Ӧ����ʽΪ___������B�Ļ�ѧʽΪ___��
��3���������еij��Ӱ���������
�ټ�������MnO2��Fe2+ת��ΪFe3+���䷴Ӧ�����ӷ���ʽΪ___��
�ڼӰ�ˮ��pH=5.6�����ɵij����Ļ�ѧʽ��__��
�ۼ�������NH4F������Ϊ���ʵ�ʣ�����������ת��Ϊ������ˮ�ij�����ȥ��
��4������Һ�к��е�������Ҫ��__��