��Ŀ����
����Ŀ���� ���и������ʣ���O2��O3 ��H2��D2��T2 ��12 C��14 C ��CH3CH2CH2CH3��(CH3)2CHCH3 �ݹ����ʮ���� ��CH3(CH2)5CH3��CH3CH2CH2CH(CH3)C2H5 (�ں���������Ӧ�����)

A����Ϊͬλ�ص���_________ �� B����Ϊͬ���칹�����________��
C����Ϊͬ�����������________�� D��ͬһ�����ʵ���_________��
�� д�����������ķ���ʽ
��1������30����ԭ�ӵ��� ��
��2������ij��������Է�������Ϊ142����������ķ���ʽΪ ��
��3������A��ͬ��ͬѹ���������ܶ���H2��36�� ��
��4��1L����D��������ȫȼ��ʱ,����ͬ��ͬѹ��15Lˮ���� ��
������ĵ���ʽΪ________��̼���֮�����Ϊ ������������ڹ����·���ȡ����Ӧ������___________�ֲ��������_________��������������д����������ڹ�������������������Ӧ�Ļ�ѧ����ʽ��____________________________________________________________��
���𰸡�
��A���� �� B���ܢ���C������ D��������
�� ��1�� C14H30 ����2��C10H22 ����3��C5H12����4��C14H30 ��
�� �� 109�� 28, ��5��CCl4�� CHBr3��Br2����CBr4��HBr
�� 109�� 28, ��5��CCl4�� CHBr3��Br2����CBr4��HBr
��������
����������� ���и������ʣ���O2��O3 ��H2��D2��T2 ��12 C��14 C ��CH3CH2CH2CH3��(CH3)2CHCH3 �ݹ����ʮ���� ��CH3(CH2)5CH3��CH3CH2CH2CH(CH3)C2H5 (�ں���������Ӧ�����)  ��A��ͬλ������������ͬ��������ͬ��һ��Ԫ�أ���Ϊͬλ�ص��Ǣ�12 C��14 C �� B��ͬ���칹��������ʽ��ͬ���ṹʽ��ͬ�����ʳ�Ϊͬ���칹�壬��Ϊͬ���칹������ܢ���C��ͬ��Ԫ����ɵIJ�ͬ������Ϊͬ������������Ϊͬ���������������� D��ͬһ�����ʵ����ڢߣ���Ϊ��A���� ��B���ܢ���C������ D���ڢ�����.��1������������ͨʽCnH2n+2��֪������30��Hԭ�ӵ�����ΪC14H30 ����Ϊ��C14H30����2��ij����ΪCnH2n+2������Է�������Ϊ14n+2=142�����n=10���������ķ���ʽΪC10H22 ����Ϊ��C10H22 ����3������A��ͬ��ͬѹ���������ܶ���H2��36��������������Է�������Ϊ72����A�ķ���ʽΪΪCnH2n+2������Է�������Ϊ14n+2=72�����n=5���������ķ���ʽΪC5H12����Ϊ��C5H12����4��1L����D��������ȫȼ��ʱ,����ͬ��ͬѹ��15Lˮ��������ô�����е���ԭ�ӵ����ʵ������������ʵ���֮��Ϊ15��2:1����ô�����е�Hԭ����ĿΪ30��������������ͨʽCnH2n+2��֪������30��Hԭ�ӵ�����ΪC14H30 ����Ϊ��C14H30����.CH4�ĵ���ʽΪ
��A��ͬλ������������ͬ��������ͬ��һ��Ԫ�أ���Ϊͬλ�ص��Ǣ�12 C��14 C �� B��ͬ���칹��������ʽ��ͬ���ṹʽ��ͬ�����ʳ�Ϊͬ���칹�壬��Ϊͬ���칹������ܢ���C��ͬ��Ԫ����ɵIJ�ͬ������Ϊͬ������������Ϊͬ���������������� D��ͬһ�����ʵ����ڢߣ���Ϊ��A���� ��B���ܢ���C������ D���ڢ�����.��1������������ͨʽCnH2n+2��֪������30��Hԭ�ӵ�����ΪC14H30 ����Ϊ��C14H30����2��ij����ΪCnH2n+2������Է�������Ϊ14n+2=142�����n=10���������ķ���ʽΪC10H22 ����Ϊ��C10H22 ����3������A��ͬ��ͬѹ���������ܶ���H2��36��������������Է�������Ϊ72����A�ķ���ʽΪΪCnH2n+2������Է�������Ϊ14n+2=72�����n=5���������ķ���ʽΪC5H12����Ϊ��C5H12����4��1L����D��������ȫȼ��ʱ,����ͬ��ͬѹ��15Lˮ��������ô�����е���ԭ�ӵ����ʵ������������ʵ���֮��Ϊ15��2:1����ô�����е�Hԭ����ĿΪ30��������������ͨʽCnH2n+2��֪������30��Hԭ�ӵ�����ΪC14H30 ����Ϊ��C14H30����.CH4�ĵ���ʽΪ ��̼���֮��ļ���Ϊ109�� 28,������������ڹ����·���ȡ����Ӧ����������һ�ȼ��顢���ȼ��顢�ȷ¡����Ȼ�̼���Ȼ�����֣�����CCl5��������������������Br2����ȡ����Ӧ�������廯̼������ʽΪ��CHBr3��Br2����CBr4��HBr����Ϊ��
��̼���֮��ļ���Ϊ109�� 28,������������ڹ����·���ȡ����Ӧ����������һ�ȼ��顢���ȼ��顢�ȷ¡����Ȼ�̼���Ȼ�����֣�����CCl5��������������������Br2����ȡ����Ӧ�������廯̼������ʽΪ��CHBr3��Br2����CBr4��HBr����Ϊ�� �� 109�� 28, ��5��CCl4�� CHBr3��Br2����CBr4��HBr��
�� 109�� 28, ��5��CCl4�� CHBr3��Br2����CBr4��HBr��

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�����Ŀ����1��п�̸ɵ����һ��һ�ε�أ����Ϊ����п���м���̼��������Χ��̼�ۣ��������̣��Ȼ�п���Ȼ�淋���ɵ������õ���ڷŵ���̲���MnOOH��������ˮ�������մ����÷ϵ�ؿ��Եõ����ֻ���ԭ�ϣ��й�������ͼ��ʾ��
�ܽ��/(g/100gˮ)
�¶�/�� ������ | 0 | 20 | 40 | 60 | 80 | 100 |
NH4Cl | 29.3 | 37.2 | 45.8 | 55.3 | 65.6 | 77.3 |
ZnCl2 | 343 | 395 | 452 | 488 | 541 | 614 |
������ | Zn(OH)2 | Fe(OH)2 | Fe(OH)3 |
Ksp����ֵ | 10-17 | 10-17 | 10-39 |
�ش��������⣺
����ͨп�̵�طŵ�ʱ��������Ҫ��ӦΪ��Zn+2NH4Cl+2MnO2��Zn(NH3)2Cl2+2MnOOH���õ���У�����������Ҫ��________��������������Ҫ��Ӧʽ�� ��
�Ӽ������ҺpH��ʹ��Һ������������Ũ�ȴﵽ mol/L�����պ���ȫ����������Ũ��С��1��10-5mol/Lʱ��������Ϊ�����ӳ�����ȫ���������Ӽ����pHΪ ��п��ʼ�������ٶ�Zn2��Ũ��Ϊ0.1mol/L����
��2���ڷ�����ѧ�г���Na2C2O4��������Һ��ɫ����Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȡ���H2SO4��Һ�У���Ӧ���£�2MnO��5C2O��16H��![]() 2Mn2������Һ��ɫ����10CO2����8H2O��
2Mn2������Һ��ɫ����10CO2����8H2O��
������W g Na2C2O4���100 mL����Һ����ȡ20.00 mL������ƿ�У�������KMnO4��ҺӦװ��__________(���ʽ����ʽ��)�ζ����С����εζ� ѡ��ָʾ����������Ҫ����������Ҫ�������жϵζ����յ��������____ ___��
�����ζ�����ʼ�������յ������ͼ��ʾ��������KMnO4�����ʵ���Ũ��Ϊ_ (�����ʽ)��
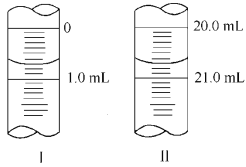
�����ζ���������������ⶨ��KMnO4��Һ��Ũ��___(�ƫ�ߡ�����ƫ�͡����䡱)��
����Ŀ��I�����ʾΪԪ�����ڱ���һ���֣�����Ԫ���������ڱ��е�λ�ã���ش��������⣺
�� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | |||
��1��д������������Ԫ��ԭ�Ӱ�1:1��ɵij���������ĵ���ʽ ��
��2���õ���ʽ��ʾ�������γɻ�����Ĺ��̣� ��
��3������������������������Ӧˮ�����������ǿ������˳��Ϊ ���û�ѧʽ��ʾ����
��4����֪���ڱ��д��ڶԽ����ƹ����������������ڱ��д��ڶԽ���λ����ѧ�������ƣ������������������Ҳ�����ԣ�д���������������������������ﷴӦ�Ļ�ѧ����ʽ ��
��5�����������������Ӱ뾶�ɴ�С��˳��Ϊ �������ӷ��ű�ʾ����
IIijͬѧ��������ͼװ����֤ͬ����Ԫ�����ʵݱ���ɡ���Ҫ֤���ǽ����ԣ�Cl��I����A�м�Ũ���ᣬB�м�KMnO4����KMnO4��Ũ���᳣���·�Ӧ������������C�мӵ��۵⻯�ػ����Һ���۲쵽C��Һ������Ϊ ����Ӧ���ӷ���ʽΪ ������֤����



