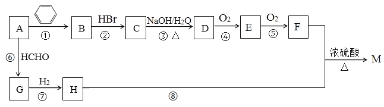
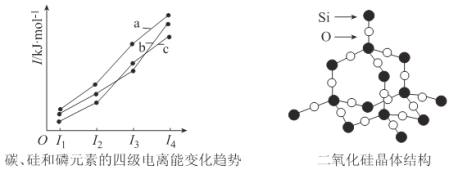
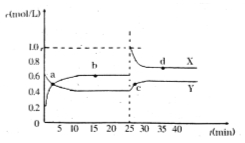
��Ŀ����
����Ŀ��W��X��Y��Z��ԭ���������������ͬһ������Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�ء�
��1��W��X������������Ӧ��ˮ������Է�Ӧ����ij���ӻ���������ӷ���ʽΪ___________��
��2��W��Y���γɻ�����W2Y���û�����ĵ���ʽΪ_______________��
��3��Y�ĵͼ�������ͨ��Z���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ___________��
��4���Ƚ�Y��Z��̬�⻯����ȶ��ԣ�__________(�û�ѧʽ��ʾ����
��5��W��X��Y��Z����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳����_______________��
���𰸡���1��Al(OH)3��OH��=AlO2����2H2O��
��2��![]() ��
��
��3��Cl2��SO2��2H2O=H2SO4��2HCl��
��4��HCl>H2S��
��5��S2����Cl����Na����Al3��
�����������������W��X��Y��Z��ԭ���������������ͬһ������Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ��, ��1��W��X������������Ӧ��ˮ������Է�Ӧ����ij���ӻ��������W��X�ǽ���Ԫ�أ�˵�����ǵ�����������Ӧˮ����ķ�Ӧ�Ǽ�֮��ķ�Ӧ������W��Na��X��Al���������ƺ�����������Ӧ�����ӷ���ʽΪAl(OH)3��OH��=AlO2����2H2O����Ϊ��Al(OH)3��OH��=AlO2����2H2O��
��2��Na��Y���γɻ�����Na2Y��˵��Y��SԪ�أ����Ƶĵ���ʽΪ![]() ����Ϊ��
������![]() ��
��
��3��Z��ClԪ�أ�����������ͨ����ˮ�з���������ԭ��Ӧ����������Ȼ��⣬��Ӧ�Ļ�ѧ����ʽΪ��Cl2��SO2��2H2O=H2SO4��2HCl����Ϊ��Cl2��SO2��2H2O=H2SO4��2HCl��
��4���ǽ�����Cl>S��������̬�⻯����ȶ���HCl>H2S����Ϊ��HCl>H2S��
��5�����Ӳ���Խ�࣬�뾶Խ������ͬʱ���˵��Խ��뾶ԽС��Na��Al��S��ClԪ�صļ����ӵİ뾶�ɴ�С��˳��Ϊ��S2����Cl����Na����Al3������Ϊ��S2����Cl����Na����Al3��.

 Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�