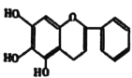
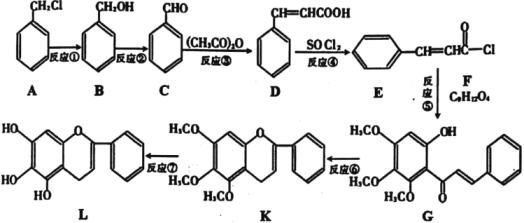
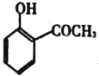
��Ŀ����
����Ŀ����������;�㷺,��¯�������ܷ�ӦΪ:Fe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g),��ش���������:
2Fe(s)+3CO2(g),��ش���������:
��1��һ���¶���,������̶����ܱ������з���������Ӧ,�����жϸ÷�Ӧ�Ѿ��ﵽƽ�����___________
A.�ܱ���������ѹǿ����
B.�ܱ������л�������ƽ��Ħ����������
C.�ܱ������л��������ܶȲ���
D.c(CO)=c(CO2)
E.Fe2O3���������ٱ仯
��2��һ���¶���,������Ӧ�Ļ�ѧƽ�ⳣ��Ϊ3.0,���¶��½�4molCO��2molFe2O3��6molCO2��5molFe�����ݻ�Ϊ2L���ܱ�������,��ʱ��Ӧ����________________��Ӧ�������(������������������������ƽ��״̬��);��Ӧ��ƽ���,�������¶�,CO��CO2�����������,������ӦΪ______________��Ӧ(��������������������)��
��3����֪:3Fe2O3(s)+CO(g)![]() 2Fe3O4(s)+CO2(g) ��H=�C47kJ/mol
2Fe3O4(s)+CO2(g) ��H=�C47kJ/mol
Fe3O4(s)+CO(g)![]() 3FeO(s)+CO2(g) ��H=+19kJ/mol
3FeO(s)+CO2(g) ��H=+19kJ/mol
FeO(s)+CO(g)![]() Fe(s)+CO2(g) ��H=�C11kJ/mol
Fe(s)+CO2(g) ��H=�C11kJ/mol
��Fe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g)�� ��H=________________��
2Fe(s)+3CO2(g)�� ��H=________________��
���𰸡�BCE �� ���� -25kJ/mol
��������
(1)A.������ͬ�����£�ѹǿ֮�ȵ����������ʵ���֮�Ƚ��з�����
B.����Ħ�������Ķ�����з�����
C.�����ܶȵĶ�����з�������
D.����ƽ��״̬�Ķ�����з������жϣ�
E.���ݻ�ѧƽ��״̬�Ķ�����з������жϣ�
(2)����Q��K�Ĺ�ϵ���з������жϣ�
(3)���ø�˹���ɽ��з����ͼ��㣻
(1)A. ��ͬ�����£�����ѹǿ֮�ȵ����������ʵ���֮�ȣ�Fe2O3��FeΪ���壬CO��CO2Ϊ���壬�ҷ�Ӧǰ������ϵ��֮����ȣ���˷�Ӧ�κ�ʱ�̣�ѹǿ������ȣ�����ܱ���������ѹǿ���䣬����˵����Ӧ�ﵽƽ�⣬��A���������⣻
B. ����M=![]() ,��Ӧǰ������ϵ��֮����ȣ����������ʵ���ʼ�ղ��䣬���ݷ�Ӧ����ʽ��������Ӧ������У���������������˵�����ƽ��Ħ���������䣬˵���÷�Ӧ�ﵽƽ�⣬��B�������⣻
,��Ӧǰ������ϵ��֮����ȣ����������ʵ���ʼ�ղ��䣬���ݷ�Ӧ����ʽ��������Ӧ������У���������������˵�����ƽ��Ħ���������䣬˵���÷�Ӧ�ﵽƽ�⣬��B�������⣻
C. �����ܶȵĶ��壬����Ϊ������䣬������������ֲ��䣬����Bѡ��������������������仯�����������ܶȱ��ֲ��䣬˵���÷�Ӧ�ﵽƽ�⣬��C�������⣻
D. ���ݻ�ѧƽ��״̬�Ķ��壬ƽ��ʱ����ֵ�Ũ�ȱ��ֲ��䣬��c(CO)=c(CO2)��������Ϊ�ﵽƽ��ı�־����D���������⣻
E. Fe2O3Ϊ��Ӧ����ŷ�Ӧ���У���������������С�������������������ٱ仯��˵���÷�Ӧ�ﵽƽ�⣬��E�������⣻
(2)Fe2O3��FeΪ���壬��˸÷�Ӧ��ѧƽ�ⳣ���ı���ʽΪK=![]() ,���¶��½�4molCO��2molFe2O3��6molCO2��5molFe�����ݻ�Ϊ2L���ܱ������У��жϷ�Ӧ���еķ�����Ҫ����Q��K֮��Ĺ�ϵ�����жϣ�Q=
,���¶��½�4molCO��2molFe2O3��6molCO2��5molFe�����ݻ�Ϊ2L���ܱ������У��жϷ�Ӧ���еķ�����Ҫ����Q��K֮��Ĺ�ϵ�����жϣ�Q=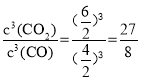 >K=3,�÷�Ӧ���淴Ӧ������У������¶ȣ�CO��CO2�����������˵��ƽ�����淴Ӧ�����ƶ���������������ԭ��������ӦΪ���ȷ�Ӧ��
>K=3,�÷�Ӧ���淴Ӧ������У������¶ȣ�CO��CO2�����������˵��ƽ�����淴Ӧ�����ƶ���������������ԭ��������ӦΪ���ȷ�Ӧ��
(3) 3Fe2O3(s)+CO(g)![]() 2Fe3O4(s)+CO2(g) ��H=�C47kJ/mol ��
2Fe3O4(s)+CO2(g) ��H=�C47kJ/mol ��
Fe3O4(s)+CO(g)![]() 3FeO(s)+CO2(g) ��H=+19kJ/mol ��
3FeO(s)+CO2(g) ��H=+19kJ/mol ��
FeO(s)+CO(g)![]() Fe(s)+CO2(g) ��H=�C11kJ/mol ��
Fe(s)+CO2(g) ��H=�C11kJ/mol ��
����Ŀ�귴Ӧ����ʽ�Լ���˹���ɿ�֪��![]() ����H=��25kJ/mol��
����H=��25kJ/mol��

����Ŀ�����ٵ����������̼���������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ������Ӧ�úʹ���̼�������仯�������������������Ҫ���塣
(1)���������� CO2 ���о�һֱ�ǿƼ����ע���ص㡣�ڴ����������� H2 ��ԭ CO2 �ǽ������ЧӦ����Ҫ�ֶ�֮һ��
��֪����H2 �� CH4 ��ȼ���ȷֱ�Ϊ 285.5 kJ/mol �� 890.0 kJ/mol��
��H2O(1)===H2O(g) ��H��+44 kJ/mol
��д��H2��ԭCO2����CH4��H2O(g)���Ȼ�ѧ����ʽ_____��
(2)CO2��CuZnO���£���ͬʱ�������µķ�ӦI��II�������Ϊ�������ЧӦ����Դ��ȱ����Ҫ�ֶΡ�
I��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)��H1����57.8kJ/mol
CH3OH(g)��H2O(g)��H1����57.8kJ/mol
II��CO2(g)��H2(g)![]() CO(g)��H2O(g)��H2��+41.2kJ/mol
CO(g)��H2O(g)��H2��+41.2kJ/mol
��������μӵķ�Ӧ����ʾƽ�ⳣ��Kpʱ�����������(B)��ƽ���ѹp(B)����������ƽ��Ũ��c(B)����ӦII��Kp=_____����֪���������ֵķ�ѹp(B)��������ѹ��������������ݡ�
��CuZnO���ڵ������£������¶�T���䣬��һ�����ܱ������У�����һ������CO2��H2����ʼ����ƽ��ʱ�������ڸ��������ʵ������±���
CO2 | H2 | CH3OH | CO | H2O(g) | ��ѹ/kPa | |
��ʼ/mol | 5.0 | 7.0 | 0 | 0 | 0 | p0 |
ƽ��/mol | n1 | n2 | p |
����ӦI��II����ƽ��ʱ��p0��1.2p�������n1/span>��_____������ʱn2��3����ӦI��ƽ�ⳣ��Kp��_____(�������λ���ú���ѹp��ʽ�ӱ�ʾ)��
(3)����β���������γɵ�ԭ��֮һ���о���������Ĵ�����������Ч�����������γɡ��ɲ���������ԭ��������4NO(g)��4NH3(g)��O2(g)![]() 4N2(g)��6H2O(g)��H��0
4N2(g)��6H2O(g)��H��0
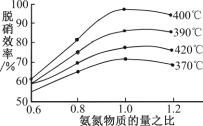
(4)����ͼʾ�ж��������Ч�ʵ����������_____��������һ��ʱ����400��ʱ������Ч���������ܵ�ԭ����_____���û���̿��ԭ��Ҳ���Դ����������ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO��������Ӧ��C(s)��2NO(g)![]() N2(g)��CO2(g)��H��0��T��ʱ����Ӧ���е���ͬʱ���ø����ʵ���Ũ�����£�
N2(g)��CO2(g)��H��0��T��ʱ����Ӧ���е���ͬʱ���ø����ʵ���Ũ�����£�
ʱ��/min | 0 | 10 | 20 | 30 | 40 | 50 |
c(NO)/molL1 | 1.0 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
c(N2)/molL1 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
c(CO2)/molL1 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
30min��ֻ�ı�ijһ�����������ϱ��������жϸı������������______����ĸ)��
A��ͨ��һ������ CO2 B��������ʵĴ��� C���ʵ���С���������
D��ͨ��һ������ NO E������һ�����Ļ���̿ F���ʵ������¶�
����Ŀ����������������ǹ�ҵ����Ҫ�Ļ���ԭ�ϣ�Ҳ��ʵ�����ﳣ���Ļ�ѧ�Լ������ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000 mol��L-1 HCl����Һ�����к͵ζ�(�÷�̪��ָʾ��)����ش��������⣺
��1���ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ_____��
��2����ʽ�ζ���������ˮϴ��������Ӧ�ý��еIJ�����_________��
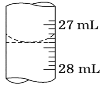
��3������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50 mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ_____��
��ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.11 | 25.10 |
2 | 25.00 | 1.56 | 33.30 |
3 | 25.00 | 0.21 | 25.22 |
��4��ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ______(������λ��Ч����)��
��5��������Щ������ʹ�ⶨ���ƫ��_____ (�����)��
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
��6���ζ��ﵽ�յ�ı�־��________��