��Ŀ����
����Ŀ���л���AΪ��Ȼ������ˮ��������C��H��N��O����Ԫ�ء�ij�о�С��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ�鲽�� | ʵ����� |
(1)��A����ʹ�������������ܶ�ԼΪ��ͬ�����¿����ܶȵ�4.6���� | A����Է�������Ϊ_____ |
(2)��26.6gA��������O2�г��ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ҡ����ȵ�ͭ��(����ÿ�����屻��ȫ����)��ǰ���߷ֱ�����12.6g��35.2g��ͨ��ͭ�����ռ���������Ϊ2.24L(��״����)�� | A�ķ���ʽΪ_______ |
(3)��ȡ13.3gA����������NaHCO3��ĩ��ȫ��Ӧ����������4.48L(��״����)�� | A�к��еĹ���������Ϊ____ |
(4)A�ĺ˴Ź���������ʾ������ҷ������Ϊ1:1:1:2:2�� | A�Ľṹ��ʽΪ_______ |
(5)������A��Ӧ����һ����Ԫ��״������Ļ�ѧ����ʽΪ_________ | |
���𰸡�133 C4H7O4N �Ȼ� ���� HOOC��CH2��CH(NH2)��COOH 2HOOC��CH2��CH(NH2)��COOH��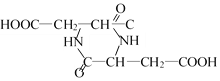 +2H2O
+2H2O
��������
��1���ܶ�����ͬ�����¿�����4.6��������Է�������ҲΪ������4.6����
��2��Ũ�������ص���������ˮ����������ʯ�����ص��������Ƕ�����̼�����������ȵ�ͭ���������dz�ȥ������е�����������ͨ��ͭ�����ռ���������Ϊ������
��3����̼�����Ʒ�Ӧ�ų����壬˵����-COOH��
��4���˴Ź���������ʾ������ҷ������Ϊ1:1:1:2:2��˵����5�е�Ч�⣬�Ҹ�����Ϊ1:1:1:2:2��
(1) A����ʹ�������������ܶ�ԼΪ��ͬ�����¿����ܶȵ�4.6��������A����Է��������ǿ���ƽ����Է���������4.6��������A����Է�������=29��4.6��133���ʴ�Ϊ��133��
(2)�ɷ�����֪�����л���ȼ�ղ���ˮ������=12.6g�����ʵ���=![]() =0.7mol��Hԭ�ӵ����ʵ���=1.4mol������������̼������=35.2g�����ʵ���=
=0.7mol��Hԭ�ӵ����ʵ���=1.4mol������������̼������=35.2g�����ʵ���=![]() =0.8mol��Cԭ�ӵ����ʵ���=0.8mol�����������ʵ���=
=0.8mol��Cԭ�ӵ����ʵ���=0.8mol�����������ʵ���=![]() =0.1mol��Nԭ�ӵ����ʵ���=0.2mol�����(1)��֪ȼ�ո��л�������ʵ���=
=0.1mol��Nԭ�ӵ����ʵ���=0.2mol�����(1)��֪ȼ�ո��л�������ʵ���=![]() =0.2mol�����Ը��л�������У�C�ĸ���=
=0.2mol�����Ը��л�������У�C�ĸ���=![]() =4��ͬ����H�ĸ���=7��N�ĸ���=1������л������ʽΪC4H7OxN����12��4+7��1+14��1+16x=133�����x=4�����Ը��л������ʽΪC4H7O4N���ʴ�Ϊ��C4H7O4N��
=4��ͬ����H�ĸ���=7��N�ĸ���=1������л������ʽΪC4H7OxN����12��4+7��1+14��1+16x=133�����x=4�����Ը��л������ʽΪC4H7O4N���ʴ�Ϊ��C4H7O4N��
(3)�л�������ʵ���=![]() =0.1mol������������̼�����ʵ���=
=0.1mol������������̼�����ʵ���=![]() =0.2mol������-COOH��NaHCO3��CO2��֪��ÿ1mol�Ȼ��ܺ�̼�����Ʒ�Ӧ����1mol������̼���壬���Բ���0.2mol������̼��Ӧ0.2mol�Ȼ������Ը��л��ﺬ2���Ȼ�����Ϊ���л���Ϊ������ˮ�������Ի���1���������ʴ�Ϊ���Ȼ���������
=0.2mol������-COOH��NaHCO3��CO2��֪��ÿ1mol�Ȼ��ܺ�̼�����Ʒ�Ӧ����1mol������̼���壬���Բ���0.2mol������̼��Ӧ0.2mol�Ȼ������Ը��л��ﺬ2���Ȼ�����Ϊ���л���Ϊ������ˮ�������Ի���1���������ʴ�Ϊ���Ȼ���������
(4)�˴Ź�������˵����5���⣬�Ҹ�����=1:1:1:2:2�����Ը��л���ṹ��ʽΪ��HOOC��CH2��CH(NH2)��COOH���ʴ�Ϊ��HOOC��CH2��CH(NH2)��COOH��
(5)�����Ӹ��л�����Է�����Ӧ������Ԫ��������Ԫ��ֻ����Ϊ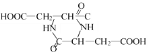 �����Է�Ӧ�ķ���ʽΪ��2HOOC��CH2��CH(NH2)��COOH��
�����Է�Ӧ�ķ���ʽΪ��2HOOC��CH2��CH(NH2)��COOH��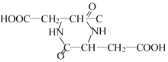 +2H2O���ʴ�Ϊ��2HOOC��CH2��CH(NH2)��COOH��
+2H2O���ʴ�Ϊ��2HOOC��CH2��CH(NH2)��COOH��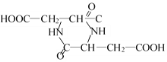 +2H2O��
+2H2O��

 ��������ϵ�д�
��������ϵ�д�����Ŀ����Ҫ��ش��������⡣
(1)���л�̬ԭ�ӻ����ӵĵ����Ų�ʽ������ʾʽ��ȷ����_______(����ţ���ͬ)��Υ���������ԭ������_____��Υ������������ԭ������_____��Υ�����ع������_______��
��Si��![]()
��Al��![]()
��Co3+����㣺![]()
��Mg2����1s22s22p6
��Sc��1s22s22p63s23p63d3
��Cr��1s22s22p63s23p63d54s1
(2)���ʣ��ټ��� ������ ��������þ �ܰ��� ����ϩ
���� | �����������ʵ���� |
�Ⱥ����Լ��ֺ��Ǽ��Լ� | ______ |
���м��Լ��ļ��Է��� | ______ |
���������м����ɴ�С��˳�� | ______ |
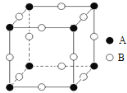
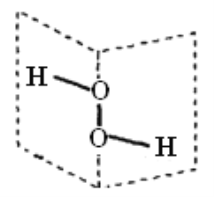
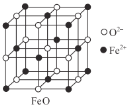
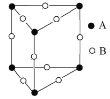
(3)��ͼFeO��������Fe2+�����Fe2+�ĸ���Ϊ__________����ͼ������A��B������������Ϊ______����ͼ������A��B������������Ϊ_________��
��. ��.
��. ��.
��.