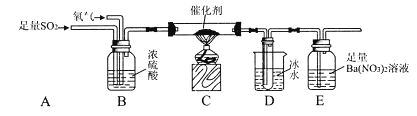
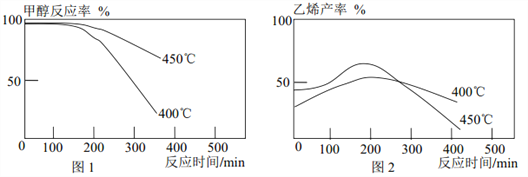
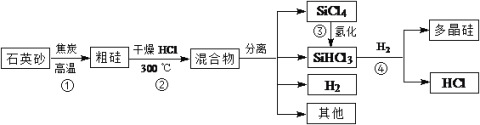
��Ŀ����
����Ŀ����1��Ǧ����������Ķ��ε�أ��ŵ�ʱ�Ļ�ѧ����ʽΪ��Pb(s)+PbO2(s)+2H2SO4(aq)��2PbSO4(s)+2H2O(l)�������طŵ�ʱ���������Һ�� SO42-����_____________(����������������)����һ��ʱ��������� 48g��ת�Ƶ���___________mol��
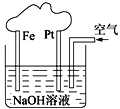
��2����ͼ�Ǽ���ȼ�ϵ��ԭ��ʾ��ͼ���ش��������⣺

����صĸ���Ϊ_____��(����a������b��)��
����ع���һ��ʱ���������Һ�� pH_____(��������������С������������)��
���𰸡��� 1 a ��С
��������
(1)���ݷŵ�ʱ�Ļ�ѧ����ʽ��Pb(s)+PbO2(s)+2H2SO4(aq)��2PbSO4(s)+2H2O(l)����д��Ǧ���صĵ缫��Ӧ�����ԭ��صĹ���ԭ���ɽⱾС�⣻
(2)��ȼ�ϵ���У�ȼ���ڸ���������O2��������ԭ���缫��ӦΪ��������2O2 + 8e- + 4H2O = 8OH-��������CH4 + 10OH- �C 8e- = CO32- + 7H2O���ܷ�Ӧ��CH4 + 2OH- + 2O2 = CO32- + 3H2O�����ڴ˻����ϽⱾС�⡣
(1)ԭ��ع���ʱ������������������SO42-�����������缫��ӦΪ��Pb + SO42- �C 2e- = PbSO4�����ص�48gΪSO42-����������ת�Ƶ��ӣ�![]() ��
��
����������1��
(2)��ȼ�ϵ���У�ȼ���ڸ�����Ӧ�����ԣ���صĸ���Ϊa��
����a��
�ڸ����ܷ�Ӧ����ع���ʱ����OH-������һ��ʱ���������Һ��pH��С��
��Ϊ����С��

����Ŀ����2L�ܱ������н��з�Ӧ��mX(g)��nY(g)![]() pZ(g)��qQ(g)��ʽ��m��n��p��qΪ��ѧ����������0��3min�ڣ����������ʵ����ı仯���±���ʾ��
pZ(g)��qQ(g)��ʽ��m��n��p��qΪ��ѧ����������0��3min�ڣ����������ʵ����ı仯���±���ʾ��
���� | X | Y | Z | Q |
��ʼ/mol | 0.7 | 1 | ||
2minĩ/mol | 0.8 | 2.7 | 0.8 | 2.7 |
3minĩ/mol | 0.8 |
��֪��2min�ڣ���(Q)��0.075mol��L��1��min��1����(Z)�æ�(Y)��1��2��
��1������ʽ��m��______��n��______��p��______��q��______��
��2��2.5min�ڣ�Q��ת����Ϊ______________��
��3�����ڸ÷�Ӧ������������Ӧ���ʵĴ�ʩ��___________
A������������� B�����߲���Q C��ͨ�����X D�������¶�
��4���÷�Ӧ�ﵽ��ѧƽ��״̬ʱ___________
A������������ѹǿ���ֲ��� B�������������ܶȱ��ֲ���
C������������ƽ��Ħ���������ֲ��� D������Ӧ�������淴Ӧ�������