��Ŀ����
A��B��C��D���ֶ�����Ԫ�أ�ԭ��������������A��C�γ�һ�ַ���X��һ��+1������Y��X��Y��ˮ��Һ�е�����������෴��BԪ�ص�ԭ�������������Ǵ�����������2������DԪ�ص�ԭ�Ӵ�����������������������������������⣨�Ի�ѧʽ��ʾ���ʻ����ӣ���
��1����298 Kʱ��1 g X������ȼ�������ȶ������ʣ�CԪ�صĵ��ʣ��ų�18.6 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
�ڰ�һ������X�����CԪ�ص�����������Ӧ��ˮ����Z�������ˮ�У���Һ��c(H+)=c(OH-)����X��Z�����ʵ���֮��n(X)��n��Z��________________��
A.����1 B.����1 C.��1
���Ի�����B4A10Ϊȼ�ϣ���DԪ�صĵ���Ϊ��ȼ������KOH��ҺΪ�������Һ���ɵ�ȼ�ϵ���У������ĵ缫��ӦʽΪ_______________________________��
��2����ϵԪ����Ԫ�����ڱ��ĵ�_______�壬��ϵԪ��Ҳ�С�ϡ����Ԫ�أ��ڲ�������Ӧ�ù㷺���ҹ��ǡ�ϡ�������������ḻ�Ĺ���֮һ���棨Ce����һ��ϡ���������仯�ϼ���+2��+4�ۣ�+4�۲���+2���ȶ�����д��Ce(SO4)2��Һ��Na2SO3��ϡH2SO4�з���������ԭ��Ӧ�����ӷ���ʽ_______________________________��
��1����4NH3(g)+3O2(g)====2N2(g)+6H2O(l);��H=-1264.8 kJ��mol-1
��B ��C4H10-26e-+34OH-====4![]() +22H2O
+22H2O
(2)��B
Ce4++![]() +H2O====Ce2++
+H2O====Ce2++![]() +2H+
+2H+
���������������Ƴ�A��HԪ�أ�B��CԪ�أ�C��NԪ�أ�D��OԪ�أ�X��NH3��Y��![]() ��Z��HNO3��B4A10��C4H10��NH3ȼ�ղ�����N2��H2O��
��Z��HNO3��B4A10��C4H10��NH3ȼ�ղ�����N2��H2O��

 �ŵ������ϵ�д�
�ŵ������ϵ�д�| A��X��Y��Z���ַ��ӵ��ȶ������� | B����������Ԫ�ع����γ����ֵ��� | C��X��Y��Z���ַ��ӵķе������� | D��X��Y��Z���ַ��Ӿ�Ϊ���Է��� |

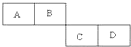 A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����
A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����