��Ŀ����
����Ŀ��W��X��Y��Z��M��G���ֶ�����Ԫ�أ�ԭ��������������W��Zͬ���壬���γ����ӻ�����ZW��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ�X����̬�⻯��ˮ��Һ�ʼ��ԡ���ش���������:
��1��Y��Ԫ�����ڱ��е�λ��Ϊ___________��
��2�� W��Y��Z��G�γɵļ����ӵİ뾶��С˳����___________(�û�ѧ���ű�ʾ)
��3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������________ (��д����)
��4��ZW�ĵ���ʽΪ___________��W2Y2�ĵ���ʽΪ______
��5��MY2��G2����ʹƷ����Һ��ɫ�����³�ѹ��������ͬ�����MY2��G2����ͬʱͨ��Ʒ����Һ������������ӷ���ʽ����ԭ��________��
��6����֪
������ | MgO | Al2O3 | MgCl2 | AlCl3 |
���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
�۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ������___________ ������ʱ�����Al2O3�������AlCl3��ԭ����___________ ��
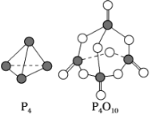
��7����������������ѧ��FulvioCacace���˻���˼��������о������N4���ӡ�N4���ӽṹ����ͼ��ʾ����֪����1 mol N��N����167 kJ����������1 mol N��N�ų�942kJ����������������Ϣ�����ݣ�����˵����ȷ����___________ ��

A��N4����һ�����ͻ����� B��N4�����۵�ߣ�Ӳ�ȴ�
C����ͬ������N4����������N2 D��1molN4ת��ΪN2������882kJ������
���𰸡��ڶ����ڵ�VIA��Cl����N3����O2����Na+O3��Cl2��ClO2��ѡ����![]()
![]() SO2+Cl2+2H2O=SO42-+2CI-+4H+����þ�۵�ߣ����ܶ��Ȼ����ǹ��ۻ��������ʱ������C
SO2+Cl2+2H2O=SO42-+2CI-+4H+����þ�۵�ߣ����ܶ��Ȼ����ǹ��ۻ��������ʱ������C
��������
W��X��Y��Z��M��G���ֶ���������Ԫ�أ�ԭ��������������XԪ�ص���̬�⻯��ˮ��Һ�ʼ��ԣ���XΪ��Ԫ�أ�Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ���MΪSԪ�ء�YΪ��Ԫ�أ�Gԭ����������Sԭ����������GΪClԪ�أ�W��Zͬ���壬���γ����ӻ�����ZW����WΪHԪ�ء�ZΪNa��
��������������WΪHԪ�ء�XΪ��Ԫ�ء�YΪ��Ԫ�ء�ZΪNaԪ�ء�MΪSԪ�ء�GΪClԪ�ء�
(1)YΪOԪ�أ���Ԫ�����ڱ��е�λ��Ϊ���ڶ����ڵ�VIA�壬�ʴ�Ϊ���ڶ����ڵ�VIA�壻
(2)���Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��Cl-��N3-��O2-��Na+���ʴ�Ϊ��Cl-��N3-��O2-��Na+��
(3)Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ���������У�O3��Cl2��ClO2���ʴ�Ϊ��O3��Cl2��ClO2��
(4)ZWΪNaH������ʽΪ![]() ��H2O2�ĵ���ʽΪ
��H2O2�ĵ���ʽΪ![]() �� �ʴ�Ϊ��
�� �ʴ�Ϊ��![]() ��
��![]() ��
��
(5)���³�ѹ��������ͬ�����SO2��Cl2����ͬʱͨ��Ʒ����Һ��������Ӧ��SO2+Cl2+2H2O=SO42-+2Cl-+4H+������ǡ�÷�Ӧ��û��ʣ�࣬Ʒ����Һ����ɫ���ʴ�Ϊ�� SO2+Cl2+2H2O=SO42-+2Cl-+4H+��
(6)��ҵ��þʱ�����MgCl2�������MgO��ԭ���ǣ�����þ�۵�ߣ����ܶࣻ����ʱ�����Al2O3�������AlCl3��ԭ���ǣ��Ȼ����ǹ��ۻ��������ʱ�����磬�ʴ�Ϊ������þ�۵�ߣ����ܶࣻ�Ȼ����ǹ��ۻ��������ʱ�����磻
(7)A��N4��NԪ����ɣ���һ�ֵ��ʣ���A����B��N4�������ڷ��Ӿ��壬���Ӿ�����۵�һ��ϵͣ�Ӳ�Ƚ�С����B����C��1molN4�����к���0.6molN-N����������2molN2���γ�2molN��N������1moN4����ת��ΪN2��ѧ���������յ�����Ϊ6��167kJ=1002kJ���γɻ�ѧ���ų�������Ϊ2��942kJ=1884kJ�����Է�Ӧ���ȣ��ų�������Ϊ1884kJ-1002kJ=882kJ��������ͬ������N4����������N2����C��ȷ��D��1molN4�����к���0.6molN-N����������2molN2���γ�2molN��N������1moN4����ת��ΪN2��ѧ���������յ�����Ϊ6��167kJ=1002kJ���γɻ�ѧ���ų�������Ϊ2��942kJ=1884kJ�����Է�Ӧ���ȣ��ų�������Ϊ1884kJ-1002kJ=882kJ����ӦΪ�ų�882kJ��������D����ѡC��