��Ŀ����
2����ҵ�Ϻϳɰ���Ӧ�Ļ�ѧ����ʽ��N2��g��+3H2��g��$?_{����}^{���¡���ѹ}$2NH3��g�������������½�һ������N2��H2�����ܱ������п�ʼ��Ӧ����1������������˵���ÿ��淴Ӧ�ﵽ��ѧƽ��״̬����AD��
A��������ѹǿ����
B��������������ܶȲ���
C����ͬʱ������3molH-H�����ѣ���6molN-H���γɣ�
D������0.1molN2��ͬʱ����0.2molNH3
��2������ʼʱ�������г���10mol•L-1��N2��15mol•L-1��H2��10min����������NH3��Ũ��Ϊ1.5mol•L-1����ʽ���㣺
��10min��N2�ķ�Ӧ���ʣ�
��H2��ת���ʣ�
���� ��1�����ݻ�ѧƽ��״̬��������𣬵���Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�ȡ��ٷֺ������䣬�Լ��ɴ�������һЩ��Ҳ�������仯������ʱҪע�⣬ѡ���жϵ������������ŷ�Ӧ�Ľ��з����仯�������������ɱ仯����ֵʱ��˵�����淴Ӧ����ƽ��״̬��
��2��N2 +3H2 ?2NH3
��ʼŨ�ȣ�mol/L�� 10 15 0
10minŨ�ȣ�mol/L�� 9.25 12.75 1.5
ת��Ũ�ȣ�mol/L�� 0.75 2.25 1.5
��v�� N2��=$\frac{0.75mol/L}{10min}$=0.075mol/��L•min�����ڦ���H2��=$\frac{2.25mol/L}{15mol/L}$��100%=15%���ɴ˽��
��� �⣺��1��A��������ѹǿ���䣬˵����������ʵ������䣬��Ӧ��ƽ��״̬������ȷ��
B��������������ܶ�ʼ�ղ��䣬��һ��ƽ�⣬�ʴ���
C����ͬʱ������3molH-H�����ѣ���6molN-H���γɣ�ֻҪ��Ӧ�����ͷ��������ϵ���ʴ���
D������0.1molN2�ĵ�Ч������0.2molNH3ͬʱ����0.2molNH3�����淴Ӧ������ȣ���ƽ��״̬������ȷ����ѡ��AD��
��2��N2 +3H2 ?2NH3
��ʼŨ�ȣ�mol/L�� 10 15 0
10minŨ�ȣ�mol/L�� 9.25 12.75 1.5
ת��Ũ�ȣ�mol/L�� 0.75 2.25 1.5
��v�� N2��=$\frac{0.75mol/L}{10min}$=0.075mol/��L•min�����ڦ���H2��=$\frac{2.25mol/L}{15mol/L}$��100%=15%��
�𣺢�10min��N2�ķ�Ӧ����Ϊ0.075mol/��L•min����
��H2��ת����15%��
���� ���⿼�黯ѧƽ��״̬���жϺ�Ӧ������ʽ���м����֪ʶ�㣬֪��ֻ�з�Ӧǰ��ı��������������Ϊƽ��״̬���ж����ݣ���Ŀ�ѶȲ���

| A�� | Cl2ͨ��Na2S��Һ�У��ܲ�������ɫ���� | |
| B�� | HCl�����ȶ��Ա�H2Sǿ | |
| C�� | HClO4�����Ա�H2SO4ǿ | |
| D�� | HCl�����Ա�H2Sǿ |
| A�� | �������飺CH2=CH2+HCl$��_{��}^{����}$ CH3CH2Cl | |
| B�� | �Ƽ���ϩ�������CH3C��CH+CO+CH3OH$\stackrel{Pd}{��}$CH2=C��CH3��COOCH3 | |
| C�� | ��CuSO4��2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CuO+H2SO4��ϡ���TCuSO4+H2O | |
| D�� | ��Cu��NO3��2��Cu+4HNO3��Ũ���TCu��NO3��2+2NO2��+2H2O |
| A�� | ��״���£�22.4LSO3����NA��SO3���� | |
| B�� | 1L0.1mol•L-1�İ�ˮ����0.1NA��OH- | |
| C�� | 1molFe2+��������H2O2��Һ��Ӧ��ת��2NA������ | |
| D�� | �����£�23g NO2����NA����ԭ�� |
| A�� | SO2��O2���ٻ��ϣ���Ӧֹͣ�� | |
| B�� | ������ѹǿ���ֲ��� | |
| C�� | SO2��O2�� SO3��Ũ����� | |
| D�� | SO2��O2�� SO3�����ʵ���֮��Ϊ2��1��2 |
| A�� | ���Ʒ�Ӧ�ų����� | B�� | �������Ƶ�Cu��OH��2����Һ��Ӧ | ||
| C�� | ��������������Һ����������Ӧ | D�� | �ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ����� |
| A�� | CH3CH2OH+CH3COOH$��_{��}^{����}$ CH3COOCH2CH3+H2O | |
| B�� | 2CH3CHO+O2 $��_{��}^{����}$2CH3COOH | |
| C�� | CH3-CH=CH2+Br2��CH3-CHBr-CH2Br | |
| D�� | 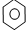 +Br2 $\stackrel{Fe}{��}$ +Br2 $\stackrel{Fe}{��}$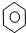 -Br+HBr -Br+HBr |
 ��
��