��Ŀ����
A��B��C��D��EΪǰ������Ԫ�أ�ԭ��������������̬Aԭ�ӵĺ������ռ��4��ԭ�ӹ����B��Cͬ���壬����ƽ�����е���B3Ũ�ȼ��ٻ����˻�Ƥ�������ӣ�D��ǰ������Ԫ���е�һ��������С��Ԫ�أ�E�ĺϽ��ǵ����������ĺϽ�

��1��E��ͬ�ڱ��е�λ���� �����̬ԭ�ӵļ����Ų�ʽΪ ��
��2��CB �����幹���� ��
�����幹���� ��
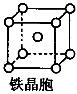
��3��D2C�ľ����ṹ��CaF2��������ͼ�����ƣ���YӦΪ �������ӷ��ţ���D2C���۵��CaF2�۵� ����ߡ������͡������Ƚϡ�����

��1��E��ͬ�ڱ��е�λ���� �����̬ԭ�ӵļ����Ų�ʽΪ ��
��2��CB
 �����幹���� ��
�����幹���� ����3��D2C�ľ����ṹ��CaF2��������ͼ�����ƣ���YӦΪ �������ӷ��ţ���D2C���۵��CaF2�۵� ����ߡ������͡������Ƚϡ�����
��1���������ڣ�1�֣� VIII�壨1�֣�; [Ar]3d64s2��2�֣�
��2���������壨2�֣�
��3��S2-��2�֣� ���ͣ�2�֣�
��2���������壨2�֣�
��3��S2-��2�֣� ���ͣ�2�֣�
���������������Ŀ���ߣ�A��̬ԭ�ӵĺ������ռ��4��ԭ�ӹ����ֻ��Ϊ1s22s22p2,AΪ̼Ԫ�ء�����ƽ�����е���B3Ũ�ȼ��ٻ����˻�Ƥ�������ӣ���BԪ��Ϊ��Ԫ�ء�D��ǰ������Ԫ���е�һ��������С��Ԫ�أ�ͬ����Ԫ�أ������ң���һ������������DԪ��Ϊ��Ԫ�ء�E�ĺϽ��ǵ����������ĺϽ�EΪ��Ԫ�ء�����ΪB��CΪͬ����Ԫ�أ�Cֻ��Ϊ��Ԫ�ء���1��E��ͬ�ڱ��е�λ���ǵ������ڣ�VIII�壬���̬ԭ�ӵļ����Ų�ʽΪ [Ar]3d64s2����2��CB
 ΪSO42-������ԭ��ΪS������4���Ҽ���û�йµ��Ӷԣ���ԭ�Ӳ�ȡsp3���ӻ����������幹�����������塣��3������ͼ����ʾ������������֪��Y:X=2:1����YΪC,XΪDԪ�ء�ͨ����Ŀ������֪��CΪ��Ԫ�أ���YӦΪS2-��D2CΪK2S����CaF2��Ϊ���Ӿ��壬�������۵�ߵ�ȡ���ڻ�����֮������Ӽ�ǿ�ȣ����Ӽ�ǿ�ȸߣ��۵�ߡ����Ӽ�ǿ�ȵľ������أ���.���Ӵ����Խ�����Ӽ�Խǿ����.���Ӱ뾶ԽС�����Ӽ�Խǿ����.�γ����Ӽ�������ԭ�ӵĵ縺�Բ�ϴ��ߣ����Ӽ���ǿ������CaF2�ĵ縺�Բ�ϴ�CaF2�ڵ�ߡ�
ΪSO42-������ԭ��ΪS������4���Ҽ���û�йµ��Ӷԣ���ԭ�Ӳ�ȡsp3���ӻ����������幹�����������塣��3������ͼ����ʾ������������֪��Y:X=2:1����YΪC,XΪDԪ�ء�ͨ����Ŀ������֪��CΪ��Ԫ�أ���YӦΪS2-��D2CΪK2S����CaF2��Ϊ���Ӿ��壬�������۵�ߵ�ȡ���ڻ�����֮������Ӽ�ǿ�ȣ����Ӽ�ǿ�ȸߣ��۵�ߡ����Ӽ�ǿ�ȵľ������أ���.���Ӵ����Խ�����Ӽ�Խǿ����.���Ӱ뾶ԽС�����Ӽ�Խǿ����.�γ����Ӽ�������ԭ�ӵĵ縺�Բ�ϴ��ߣ����Ӽ���ǿ������CaF2�ĵ縺�Բ�ϴ�CaF2�ڵ�ߡ�
��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
 3Na2O��2Fe��9N2����
3Na2O��2Fe��9N2����



 ������Ŀ֮��Ϊ ��
������Ŀ֮��Ϊ ��