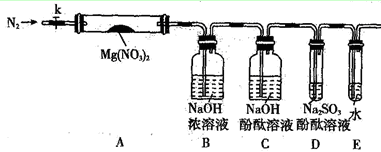
��Ŀ����
�����Ƕ���Ҫ�ǽ������仯��������ۣ�����Ҫ��ش����⣺
��1��ʵ����ʢװNaOH��Һ���Լ�ƿ�����ò�������Ӧ�����������Է�ֹ������Ӧ��
�����ӷ���ʽ����
��2������һ����Ҫ�Ļ�����Ʒ���ܶȱȿ��� �����С��������ҵ���Ʊ������Ļ�ѧ����ʽΪ ��
��3����ҵ����ȡƯ�۵ķ�Ӧ��ѧ����ʽΪ ��
��4��ŨH2SO4��������������������Ϊ������____________�������������Ƿ����ձ��У����뼸��ˮ��������ȡ�Ȼ���������Ũ���ᣬѸ�ٽ��裬�ų��������ȣ�ͬʱ�۲쵽������ڣ�������ͣ����ų��д̼�����ζ�����塣��ش�
�����̼�����ζ����Ļ�ѧ����ʽΪ ��
��5��ͭ��ϡ���ᷴӦ�����ӷ���ʽ�� �����μӷ�Ӧ��Cu����Ϊ6.4g������NO����____________L����״���£�����ת�Ƶ������ʵ���Ϊ mol������ԭ����δ����ԭ��HNO3���ʵ���֮��Ϊ ��
��1��SiO2��2OH��=SiO32����H2O ��2��С��N2��3H2 2NH3 ��3��2Cl2��2Ca(OH)2=CaCl2��Ca(ClO)2��2H2O ��4����ˮ�ԣ�C��2H2SO4��Ũ��
2NH3 ��3��2Cl2��2Ca(OH)2=CaCl2��Ca(ClO)2��2H2O ��4����ˮ�ԣ�C��2H2SO4��Ũ�� CO2����2SO2����2H2O (5)3Cu��8H����2NO3��=3Cu2����2NO����4H2O 1.49L����1.5L�� 0.2mol 1:3
CO2����2SO2����2H2O (5)3Cu��8H����2NO3��=3Cu2����2NO����4H2O 1.49L����1.5L�� 0.2mol 1:3
����

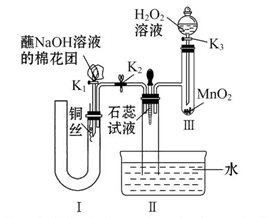
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� Na2SO3�dz��õĿ�������
��1��ʵ����ͨ����Ũ���ᣨ1��1����Na2SO3���Ʊ�SO2���壬
��Ӧ����ʽΪ�� ���Ʊ���SO2������ͨ������ˮ���������и�����ܸ���SO2������ǣ� ��
A.Ũ���� B.��ʯ�� C.��ˮCaCl2
��2�� ����SO2����ͨ��NaOH��Һ�пɵ�NaOH��Na2SO3�Ļ����Һ����û����Һ�м���������ˮ������Һ��Ϊ��ɫ��������Һ��Br2��Na2SO3����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ______________��
��3����Ӧ�����Һ����SO32����SO42����Br����OH���������ӣ�����д��������SO32����SO42����Br����ʵ�鱨�棻
��ѡ�Լ���2 mol��L��1HCl��1 mol��L��1H2SO4��1mol��L��1HNO3��1 mol��L��1BaCl2��
1 mol��L��1Ba(NO3)2��0.1 mol��L��1AgNO3��CCl4���������Ʊ�����ˮ�����Ʊ�����ˮ��
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | ȡ��������Һ���Թ�A�У��μ�2 mol��L��1HCl����Һ�����ԣ����뼸��________(���Լ�)���� | ________��֤������Һ�к�SO32- |
| ����� | ��ȡ��������Һ���Թ�B�У����� ���ٵμ����� 1 mol��L��1 BaCl2��Һ | |
| ����� | ��ȡ��������Һ���Թ�C�У� �������ú�۲���ɫ | ��Һ�ֲ㣬�ϲ�Һ��ʳȺ�ɫ��֤������Һ�к�Br- |
 Na2S2O3
Na2S2O3
 2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��
2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��

 �����ܺ���CO
�����ܺ���CO
 �����ӷ���ʽΪ_____________________________________��
�����ӷ���ʽΪ_____________________________________�� 2KNO2��O2��
2KNO2��O2�� 2CuO��4NO2����O2��
2CuO��4NO2����O2�� 2Ag��2NO2����O2��
2Ag��2NO2����O2��