��Ŀ����
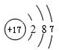
16��ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ����������γɶ���ԭ�Ӹ�����Ϊ1��1�Ļ�������еļס��ҡ�������4�ֻ������������������ӻ����A��B��ԭ������֮����Ԫ��D��һ�룮B������ɵ����ʵı�ҪԪ�أ�Ҳ��ijЩ���ʵ����Ԫ�أ�ֻ��CΪ����Ԫ�أ���ɫ��Ӧ�ʻ�ɫ����ش��������⣺��1��Ԫ��E ��ԭ�ӽṹʾ��ͼΪ
 ��
����2��B��D�γɵĻ�����ף�����Է���������170��190֮�䣬��D����������ԼΪ70%����û�����Ļ�ѧʽΪN4S4��
��3��A��B�γɵĻ�������ΪB4A4����һ�������¿��Ե�����������ӣ�����һ��Ϊ10e-�����ӣ�������һ�������µĵ��뷽��ʽΪN4H4=NH4++N3-��
��4��������C2D��Һ�ڿ����г��ڷ��û�������ɱ�C2D2���û�ѧ����ʽ��ʾ�ù���4Na2S+O2+2H2O=4NaOH+2Na2S2��
��5��D��E�γɵĻ����ﶡ������ԭ�Ӿ�����8�����ȶ��ṹ���û�����ĵ���ʽΪ
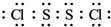 ����������ˮ���ҷ�Ӧ�������д̼�����ζ�Ļ������X��Y��ͬʱ���й��嵥�ʲ�������֪X����ʹƷ����ɫ�����壬Y�ڱ�״̬�µ��ܶ�Ϊ1.63g/L���û�����������H2���ܶ�Ϊ21���������̶�Ӧ�Ļ�ѧ����ʽΪ2S2Cl2+2H2O=SO2��+4HCl+3S����
����������ˮ���ҷ�Ӧ�������д̼�����ζ�Ļ������X��Y��ͬʱ���й��嵥�ʲ�������֪X����ʹƷ����ɫ�����壬Y�ڱ�״̬�µ��ܶ�Ϊ1.63g/L���û�����������H2���ܶ�Ϊ21���������̶�Ӧ�Ļ�ѧ����ʽΪ2S2Cl2+2H2O=SO2��+4HCl+3S����ij�о�С��ͬѧΪȷ�������X��Y���������Y �Ĵ��ڣ���Ʒ������£��Ѹ����X��Y������NH3��ϣ����ְ��̣���֤����Y���壬����Ϊ�˷�����ȷ�����ȷ������ȷ�����������Ǹ���Ķ��������백����Ӧ����������Ȼ����백����Ӧ���ɰ��̣�
���� ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ�ֻ��CΪ����Ԫ�أ���ɫ��Ӧ�ʻ�ɫ����CΪNa��B������ɵ����ʵı�ҪԪ�أ�Ҳ��ijЩ���ʵ����Ԫ�أ�BΪNԪ�أ�A��B��ԭ������֮����Ԫ��D��һ�룬��A���ڵڶ����ڣ�A��Bԭ������֮����СΪ10��D��ԭ��������СΪ20��D���Ƕ�����Ԫ�أ���AΪHԪ�أ�DΪSԪ�أ�EΪCl��
��1��EΪClԪ�أ�ԭ�Ӻ�����17�����ӣ���3�����Ӳ㣬���������Ϊ2��8��7��
��2��N��S��Ԫ����ɵĻ����������Ԫ��������������Է���������Χȷ��Sԭ����Ŀ����������S��Nԭ����Ŀ֮��Ϊ1��1������ȷ������ʽ��
��3��A��B�γɵĻ�������ΪN4H4����һ�������¿��Ե�����������ӣ�����һ��Ϊ10e-�����ӣ�ӦΪNH4+�������������ΪN3-��
��4��������Na2S��Һ�ڿ����г��ڷ��û��������Na2S2����Ӧ��SԪ�ػ��ϼ����ߣ�ֻ������Ԫ�ػ��ϼ۽��ͣ�Ӧ��NaOH���ɣ�
��5��D��E�γɵĻ����ﶡ������ԭ�Ӿ�����8�����ȶ��ṹ��Sԭ���������Ҫ2�����ӣ�Clԭ���������Ҫ1�����ӣ���DΪS2Cl2��
��������ˮ���ҷ�Ӧ�������д̼�����ζ�Ļ������X��Y��ͬʱ���й��嵥�ʲ��������嵥��ΪS��X��ʹƷ����Һ��ɫ�����壬Y�ڱ�״̬�µ��ܶ�Ϊ1.63g/L��Mr��Y��=1.63��22.4=36.5����XΪSO2��YΪHCl���û�����������H2���ܶ�Ϊ21���������ƽ����Է�������Ϊ42����ʮ�ֽ��淨��֪��SO2��HCl�����ʵ���֮��Ϊ��42-36.5������64-42��=1��4��������ƽ��д��ѧ����ʽ��
����Ķ��������백����Ӧ����������Ȼ����백����Ӧ��
��� �⣺ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ�ֻ��CΪ����Ԫ�أ���ɫ��Ӧ�ʻ�ɫ����CΪNa��B������ɵ����ʵı�ҪԪ�أ�Ҳ��ijЩ���ʵ����Ԫ�أ�BΪNԪ�أ�A��B��ԭ������֮����Ԫ��D��һ�룬��A���ڵڶ����ڣ�A��Bԭ������֮����СΪ10��D��ԭ��������СΪ20��D���Ƕ�����Ԫ�أ���AΪHԪ�أ�DΪSԪ�أ�EΪCl��
��1��EΪClԪ�أ�ԭ�Ӻ�����17�����ӣ���3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��N��S��Ԫ����ɵĻ����������Է���������170��190֮�䣬��D����������ԼΪ70%���������Sԭ����Ŀ��$\frac{190��70%}{32}$��N��S����$\frac{170��70%}{32}$����N��S��=4����������S��Nԭ����Ŀ֮��Ϊ1��1�����ΪN4S4���ʴ�Ϊ��N4S4��
��3����ΪN4H4����һ�������¿��Ե�����������ӣ�����һ��Ϊ10e-�����ӣ�ӦΪNH4+�������������ΪN3-���ʵ��뷽��ʽΪ��N4H4=NH4++N3-���ʴ�Ϊ��N4H4=NH4++N3-��
��4��������Na2S��Һ�ڿ����г��ڷ��û��������Na2S2����Ӧ��SԪ�ػ��ϼ����ߣ�ֻ������Ԫ�ػ��ϼ۽��ͣ�Ӧ��NaOH���ɣ���Ӧ����ʽΪ��4Na2S+O2+2H2O=4NaOH+2Na2S2��
�ʴ�Ϊ��4Na2S+O2+2H2O=4NaOH+2Na2S2��
��5��D��E�γɵĻ����ﶡ������ԭ�Ӿ�����8�����ȶ��ṹ��Sԭ���������Ҫ2�����ӣ�Clԭ���������Ҫ1�����ӣ���DΪS2Cl2��������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Sԭ����Cl֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ��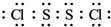 ��
��
��������ˮ���ҷ�Ӧ�������д̼�����ζ�Ļ������X��Y��ͬʱ���й��嵥�ʲ��������嵥��ΪS��X��ʹƷ����Һ��ɫ�����壬Y�ڱ�״̬�µ��ܶ�Ϊ1.63g/L��Mr��Y��=1.63��22.4=36.5����XΪSO2��YΪHCl���û�����������H2���ܶ�Ϊ21���������ƽ����Է�������Ϊ42����ʮ�ֽ��淨��֪��SO2��HCl�����ʵ���֮��Ϊ��42-36.5������64-42��=1��4����Ӧ����ʽΪ��2S2Cl2+2H2O=SO2��+4HCl+3S����
����Ķ��������백����Ӧ����������Ȼ����백����Ӧ���ɰ��̣���ʵ�鷽����ȷ��
�ʴ�Ϊ��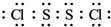 ��2S2Cl2+2H2O=SO2��+4HCl+3S������ȷ������Ķ��������백����Ӧ����������Ȼ����백����Ӧ���ɰ��̣�
��2S2Cl2+2H2O=SO2��+4HCl+3S������ȷ������Ķ��������백����Ӧ����������Ȼ����백����Ӧ���ɰ��̣�
���� ���⿼��Ԫ�ػ������ƶϣ��漰���ʾ�������ѧ�������ʣ�ע�������������е���Ϣ���з�����𣬲��ؿ���ѧ���ۺ�����֪ʶ���������Ѷ��еȣ�

| A�� | 2.5 mol | B�� | 4.5 mol | C�� | 5mol | D�� | 7.5 mol |
| A�� | SO2����Ư���ԣ���ʹ��ɫʯ����Һ��ɫ | |
| B�� | ������������þ���������ͻ���� | |
| C�� | ���ô������м����ۻ��������ƹ��� | |
| D�� | ����ˮ�е���ֲ���ͣ����Ͳ�����ɫ��˵���岻������֬ |
��������

| A�� | �ó����ʯ��ˮ�ɼ���NaHCO3��Na2CO3 | |
| B�� | ��Ԫ���ڵڢۡ����б��������ڵڢ��б���ԭ | |
| C�� | ��ҵ��һ���ý���������ˮMgCl2��Ӧ��ȡMg���� | |
| D�� | ��ˮ�л����е�Ԫ�أ�ֻ�轫��ˮ�еĵ������Ϳ��Եõ��ⵥ�� |
| A�� | ����ƿ����Ͳ�Ͼ������¶ȣ�����Ͳ������ƿ���ޡ�0���̶� | |
| B�� | �ö��������ɼ�������Һ����������Һ�͵�����Һ | |
| C�� | �ýྻ������պȡ������Һ�����ھƾ��������գ�����ɫ�ܲ����۲�K����ɫ | |
| D�� | ����ij��Һ�Ƿ���SO42-ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� |
| A�� | ͭ������FeCl3��Һ�γɵ�ԭ��أ�ͭ���ų����� | |
| B�� | �����������Һ���ǵ��ˮ��������ҺpH���� | |
| C�� | ���������������ӦΪ��O2+2H2O+4e-�T4OH- | |
| D�� | ��ҵ�ϵ�ⱥ��ʳ��ˮ��������ӦΪ��2H++2e-�TH2�� |